By : James W Zubrick
Email: j.zubrick@hvcc.edu
Sublimation occurs when you heat a solid and it turns directly into a vapor. It does not pass GO nor does it turn into a liquid. If you reverse the process — cool the vapor so that it turns back into a solid—you’ve condensed the vapor. Use the unique word, sublime, for the direct conversion of solid to vapor. Condense can refer to either vapor-to-solid or vapor-to-liquid conversions.
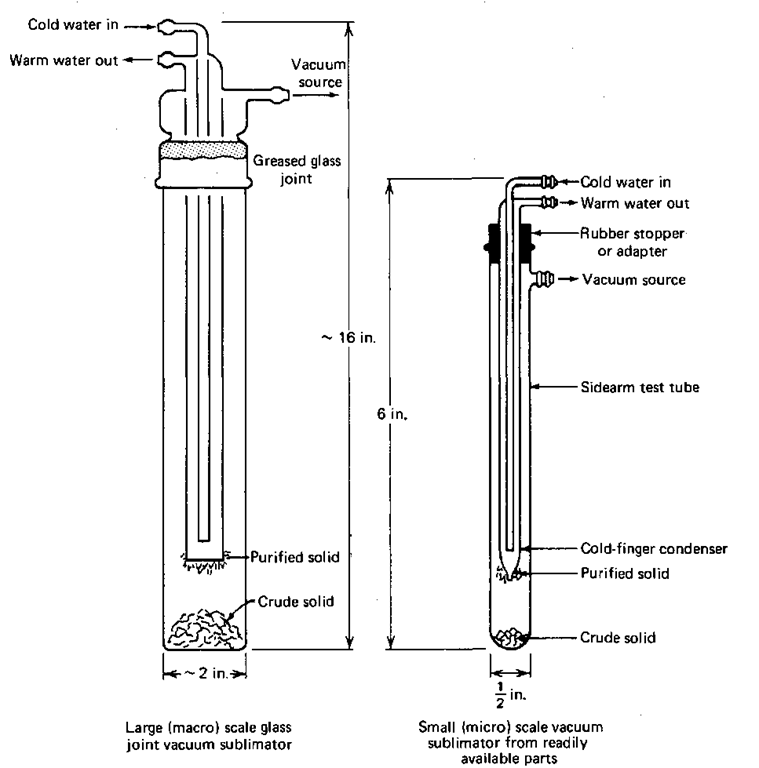
Figure 88 shows two forms of sublimation apparatus. Note all the similarities. Cold water goes in and down into a cold finger upon which the vapors from the crystals condense. The differences are that one is larger and has a ground glass joint. The sidearm test tube with cold-finger condenser is much smaller. To use them,
1. Put the crude solid into the bottom of the sublimator. How much crude solid? This is rather tricky. You certainly don’t want to start with so much that it touches the cold finger. And since as the purified solid condenses on the cold finger it begins to grow down to touch the crude solid, there has to be really quite a bit of room. I suggest that you see your instructor, who may want only a small amount purified.
Fig. 88 King-size and miniature sublimation apparatus.
2. Put the cold finger into the bottom of the sublimator. Don’t let the clean cold finger touch the crude solid. If you have the sublimator with the ground glass joint, lightly (and I mean lightly) grease the joint. Remember that greased glass joints should NOT be clear all the way down the joint.
3. Attach the hoses. Cold water goes in the center tube, pushing the warmer water out the side tube. Start the cooling water. Be careful!
4. If you’re going to pull a vacuum in the sublimator, do it now. If the vacuum source is a water aspirator, put a water trap between the aspirator and the sublimator. Otherwise you may get depressed if, during a sudden pressure drop, water backs up and fills your sublimator. Also, start the vacuum slowly. If not, air, entrained in your solid, comes rushing out and blows the crude product all over the sublimator, like popcorn.
5. When everything has settled down, slowly begin to heat the bottom of the sublimator, if necessary. You might see vapors coming off the solid. Eventually, you’ll see crystals of purified solid form on the cold finger. Since you’ll work with different substances, different methods of heating will have to be used. Ask your instructor.
6. Now the tricky parts. You’ve let the sublimator cool. If you’ve a vacuum in the sublimator, carefully—very carefully—introduce air into the device. A sudden inrush of air, and PLOP! Your purified crystals are just so much yesterday’s leftovers. Start again.
7. Now again, carefully—very carefully—remove the cold finger, with your pristine product clinging tenuously to the smooth glass surface, without a lot of bonking and shaking. Otherwise, PLOP! et cetera, et cetera, et cetera. Clean up and start again.