Filtration
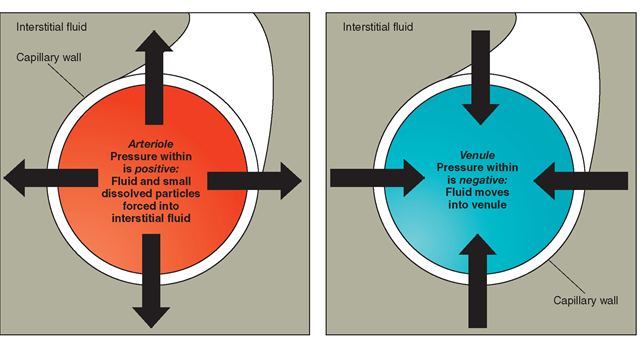
Filtration is the transport of water and dissolved materials through a membrane from an area of higher pressure to an area of lower pressure. It operates somewhat like a sieve. Filtration requires mechanical pressure. Liquids and solutes are passed through holes in a membrane. The size of the holes and the differences in pressures (mechanical force) on each side determine the amount of filtration. Filtration can be thought of as a “pushing pressure” because it pushes water and solutes through a membrane from a higher pressure area to a lower pressure area. Figure 17-5 shows filtration through an arteriole and a venule. Filtration is common in the body. Blood pressure (a mechanical force) pushes water and small dissolved particles, such as sugars and salts, through capillary walls into the interstitial fluid. The larger blood cells and proteins are too large to be pushed through and remain inside the capillaries.
Active Transport
Active transport mechanisms require specific enzymes and an energy expenditure in the form of adenosine triphosphate (ATP). Active transport processes can move solutes “uphill,” against the normal rules of concentration and pressure. Specific molecules outside a cell, even if they are fewer in number, can be assisted into the cell, even though a greater concentration already exists in the cell.
The best example of active transport is the sodium-potassium pump. More sodium ions exist outside the cell than inside. The natural tendency would be for sodium to diffuse across the cellular membrane into the cell. If this process continued unchecked, however, the homeostatic mechanism (which governs nerve transmissions and muscle contractions) would go berserk. Active transport mechanisms figuratively “pump out” sodium ions from inside the cell, while transporting potassium into the cell. Another example of active transport involves the transport of glucose and amino acids (proteins) into cells lining the small intestine. The intestinal cells use ATP to create this active transport, even if the concentration of the solutes would seem to require transport in the opposite direction.
Key Concept Passive transport does not need energy; active transport requires energy Hydrostatic pressure is the pressure exerted by a stationary liquid or fluid in a state of equilibrium. Hydrostatic mechanisms respond to changes in ECF; gains must equal losses.
FIGURE 17-5 · Filtration.
FLUID AND ELECTROLYTE BALANCE
Fluid and electrolyte balance is vital for proper functioning of all body systems. The body must maintain the correct proportion of fluids to solutes in each compartment. This balance is dependent on:
• Cell membrane permeability, as described earlier
• Osmolarity—the property of particles in a solution to dissociate into ions
• Electroneutrality—the balance of positive and negative charges
The body compensates for imbalances immediately. For example, if excess carbon dioxide (CO2) accumulates, a person breathes faster to take in more oxygen. If the respiratory system cannot handle the imbalance, chemical buffers attempt to achieve a balance. Additionally, the kidneys can adjust the pH of the ECF. Numerous hormones also act to influence fluid and electrolyte retention and excretion. Electrolytes may be lost through vomiting, diarrhea, or hemorrhage, thus upsetting the body’s fluid and electrolyte balance. Tests to determine disorders in electrolyte balance include blood tests, such as the blood urea nitrogen [BUN], the complete blood count [CBC], and evaluation of arterial blood gases [ABG], as well as urinalysis [UA].
Water Intake and Output
The body conserves and reuses water as much as possible. The kidneys, and to some extent the intestines, continuously filter and recycle water. Each day, a person must take in approximately the same amount of fluid as he or she loses. The body can survive many days without food, but only a few days without water. The term health professionals use as an external monitor of fluid balance is intake and output. Accurate intake and output records and daily weights are vital when caring for clients with fluid deficits or excesses. Table 17-4 gives sources and approximate amounts for human intake and output.
Intake refers to water and other fluids taken into the body daily. Water is obtained from three sources: liquid intake (by mouth or by a method such as an IV), as a result of food metabolism, and as the end product of cellular respiration. The average adult takes in approximately 2,000 to 3,000 mL (milliliters) per day (a little more than 2 to 3 quarts). To avoid an overload of water in the body, approximately the same amount that is taken into the body must be put out. Water output occurs normally through the kidneys, in sweat, as water vapor from the lungs, and in feces. Sweat may be sensible (visible, able to be sensed) or insensible (not perceptible to the senses) water loss. Minute amounts of fluid are also lost through vaginal secretions.
Many factors influence water loss. For example, water output increases with exercise, fever, some medications, and certain diseases. Ill individuals may need more fluids because of excess drainage from wounds, vomiting, or bleeding. A fever can cause a person to use about four times the amount of fluids that he or she would need normally. Diaphoresis (profuse sweating) can cause considerable fluid loss. Each form of fluid loss will also alter the body’s electrolyte concentrations.
TABLE 17-4. Normal Water Balances (Intake and Output)
|
INTAKE |
OUTPUT |
||
|
SOURCE |
AMOUNT |
SOURCE |
AMOUNT |
|
Liquids |
I,200 mL |
Urine |
I,500 mL |
|
Foods |
1,000 mL |
Skin* |
500 mL |
|
Metabolism |
300 mL |
Lungs* |
300 mL |
|
Feces |
200 mL |
||
|
TOTAL |
2,500 mL |
TOTAL |
2,500 mL |
*Often referred to as "insensible water losses."
Special Considerations: LIFESPAN
Adolescents are at risk for fluid and electrolyte imbalances due to excessive exercise, poor fluid intake during or after exercise, excessive use of salt and high-sodium foods and soft drinks, and following fad diets or diets lacking important nutrients—especially iron and calcium. Bulimia and anorexia are also considerations.
Key Concept In special situations, a much greater volume of fluids must be consumed, such as in the nursing mother who must offset the amount of fluid output in the breast milk.
ACID-BASE BALANCE
Acid-base balance is another important aspect of homeostasis. In the discussion on ionization, you learned that chemical compounds that break up into ions are called electrolytes and that these electrolytes can combine and recombine to form acids, bases, and salts. The body needs many of these substances to regulate acid-base levels and coordinate water balance. For example, it needs sodium to maintain the electrical potential across cell membranes and to maintain acid-base balance. Potassium acts within the cells in much the same way that sodium acts outside the cells. Chlorine plays a major role in acid-base balance because of its production of hydrochloric acid (HCl). Water contains equal components of both an acid (the hydrogen ion or H+) and a base (the hydroxyl ion or OH). Pure water is considered a neutral solution.
Acids, Bases, and Salts
An acid is one type of compound that contains the hydrogen ion (H+). A base (also known as an alkali) is a compound that contains the hydroxyl ion (OH ). A salt is a combination of a base and an acid and is created when the positive ions (usually a mineral) of a base replace the positive hydrogen ions of an acid. A salt is an electrolyte made up of a cation (other than hydrogen) and an anion (other than hydroxyl). For example, hydrochloric acid (HCl) can combine with the base sodium hydroxide (NaOH) to yield water and table salt. Thus, HCl + NaOH = HOH + NaCl. (Or an acid plus a base yields water (HOH, H2O) and a salt.)
The body contains several important salts: sodium chloride (NaCl), potassium chloride (KCl), calcium chloride (CaCl2), calcium carbonate (CaCO3), calcium phosphate (Ca3[PO4]2), and sodium sulfate (Na2SO4).
Potential of Hydrogen (pH)
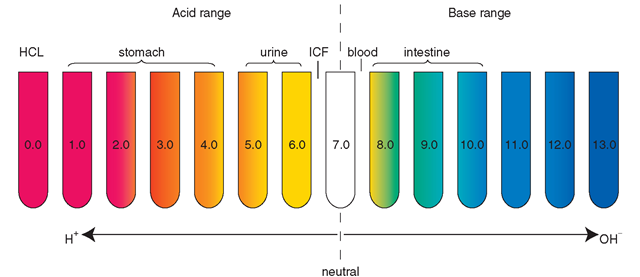
The symbol pH refers to the potential or power (p) of hydrogen ion (H+) concentration within a solution. The pH scale ranges from 0 to 14. Pure water, which is neutral, has a pH of 7. If the pH number is lower than 7, the solution is an acid. Vinegar (pH 2-3.5), lemon juice (pH 2.3), wine (pH 3.5), black coffee (pH 5.0), and tomato juice (pH 4.1) are common household acids. Acids contain more hydrogen ions than bases.
If the pH is greater than 7, a solution is basic (alkaline). The pH of ICF is approximately 6.8 to 7.0; the pH of ECF is 7.35 to 7.45. Baking soda (pH 12), toothpaste (pH 9.9), milk of magnesia (pH 10.5), detergents, and oven cleaners are common alkaline products. Basic solutions contain more hydroxyl (OH) ions than acids.
Key Concept A common remedy for excess stomach acid is baking soda (sodium bicarbonate), which is alkaline (a base).
Fluids outside the body may be strongly acidic or alkaline and not harm the body. For example, lemon juice is highly acidic (pH = 2) and sodium bicarbonate (baking soda) is highly alkaline, but they are not harmful to the body. On the other hand, some substances, either in the environment or in the body, such as hydrochloric acid (HCl, pH 0.0) could be very harmful. Figure 17-6 shows the pH of some body fluids.
Key Concept Low pH = high number of hydrogen ions; high pH = low number of hydrogen ions. Correct body fluid pH is vital because pH affects both the composition and function of proteins.
A change in the pH of a solution by one pH unit means a tenfold change in hydrogen ion concentration. For example, a substance with a pH of 5 contains 10 times more hydrogen ions than a substance with a pH of 6 (1 unit = 101). A pH of 5 means that a substance has 100 times more hydrogen ions than a substance with a pH of 7 (2 units = 102). Understanding the impact of changes in pH on the body is extremely important. For example, the pH of blood and lymph (ECF) is normally slightly alkaline, about 7.35 to 7.45. The body must maintain the slightly alkaline pH of blood within this narrow range because a decrease or increase of only one pH unit would result in chemical disaster. One of the body’s normal negative feedback mechanisms is continually correcting the body’s tendency to develop acidosis (too many hydrogen atoms) by returning the serum pH back into its alkaline state. The lungs and kidneys are the organs of the body that are the most involved in H+ regulation.
FIGURE 17-6 · The pH scale measures degree of acidity (H+ ion concentration) or alkalinity (OH ion concentration).
Key Concept Regulation of H + levels in the body is a function of kidney excretion, buffer systems, and respiration effectiveness.
Buffers
Chemical reactions occurring constantly in the body release many acidic or basic ions. Because of the constant changes in this mixture, a buffering or stabilizing system must exist to prevent pH imbalance. A buffer is a chemical system set up to resist changes, particularly in hydrogen ion levels. Buffering reactions, which can occur in less than a second, constantly alter acids and bases, to maintain a correct ratio. The body has many buffer systems related to maintaining homeostasis. Some buffer systems use phosphates; for example, phosphates in the kidneys buffer urine. Protein buffers can release H+ in hemoglobin; other special amino acids also serve as buffers. One of the major buffer systems is the bicarbonate buffer system discussed below.
The Bicarbonate Buffer System
Sodium bicarbonate has a pH of 8.4 and is a base (NaHCO3, baking soda). Carbonic acid (H2CO3) is a weak acid. These are the body’s major chemical buffers. Because of its tendency toward acidosis, the body has a greater need for sodium bicarbonate. In fact, the body contains 20 times more sodium bicarbonate than carbonic acid.
Carbon Dioxide. Chemical buffers are clinically significant, particularly in respiratory illnesses. Carbon dioxide (CO2) is a potential acid because, once dissolved in water, it becomes carbonic acid, H2CO3 (CO2 + H2O = H2CO3). When CO2 increases (as in a client with chronic obstructive pulmonary disease [COPD]), the carbonic acid content also increases. The opposite can occur in hyperventilation— where CO2 decreases, resulting in a decrease of carbonic acid. Therefore, much of the body’s acid is controlled by the respiratory system, specifically the lungs. The major compound controlled by the lungs is CO2. The respiratory system can very rapidly compensate for too much acid or too little acid by increasing or decreasing the respiratory rate, thereby altering the level of CO2. When considering respiratory acidosis or alkalosis, think about the lungs and how they compensate for imbalances, using carbon dioxide (CO2) dissolved in the blood.
Bicarbonate. The kidneys regulate the bicarbonate level in the ECF by conserving or excreting bicarbonate ions within the renal tubules. Bicarbonate levels are vital in maintaining acid-base balance. Bicarbonate ions (HCO3) are basic (alkaline) components in the body, and the kidneys are key in regulating the amount of bicarbonate in the body. If there is too much or too little base in the body, one way the body compensates is by excreting more or fewer bicarbonate ions via the kidneys. When considering acid-base balance, remember the utilization of bicarbonate ions by the kidneys. When pH drops, bicarbonate ions accept H+ ions, forming carbonic acid (H2CO3); when pH rises, carbonic acid donates H+ ions, creating a base (HCO3). These are vital factors in preventing severe acidosis or alkalosis.
Key Concept Consider:
Lungs-Acid-C02 —» H2C03
Kidneys-Base-HCO3~ ions
Major mechanisms to control acid-base balance:
• The kidneys excrete ammonia (NH3) in the form of ammonium (NH4), in an effort to balance hydrogen ions
• pH buffers: bicarbonate-base carbon dioxide-acid
• Excretion of CO2 (constantly produced by the cells)
• Hydrogen ions are making constant chemical changes
Measurement of Arterial Blood Gases. Arterial blood gases (ABGs) are measured in a laboratory test to determine the extent of compensation by the buffer system.
TABLE 17-5. Approximate Normal Arterial Blood Gas Values
|
ABBREVIATION |
NORMAL RANGE |
DEFINITION |
|
pH |
7.35-7.45 |
Reflects the hydrogen ion concentration in arterial blood Acidosis: <7.37 Alkalosis: >7.43 |
|
PaCO2 |
35-45 mm Hg |
Reflects partial pressure of carbon dioxide in arterial blood Hypocapnia: low partial pressure of carbon dioxide in arterial blood, <36 mm Hg Hypercapnia: high partial pressure of carbon dioxide in arterial blood, >44 mm Hg |
|
PaO2 |
80-100 mm Hg |
Partial pressure of O2 in arterial blood |
|
hco3~ |
22-26 mEq/L |
Amount of bicarbonate in arterial blood |
Hg, mercury (as a measurement indicator).
The pH level and amounts of specific gases in the blood indicate if there is more acid or base and their associated values. The normal range for ABGs is used as a guide, and determination of disorders is often based on the blood pH. If the blood is basic, the HCO3~ level is considered because the kidneys regulate bicarbonate ion levels. If the blood is acidic, the PaCO2 (partial pressure of carbon dioxide in arterial blood) is assessed because the lungs regulate the majority of acid. Table 17-5 shows normal ABG values. Table 17-6 illustrates acidosis and alkalosis in relationship to pH, CO2, and HCO3~ values. The distinction between metabolic and respiratory states is shown.
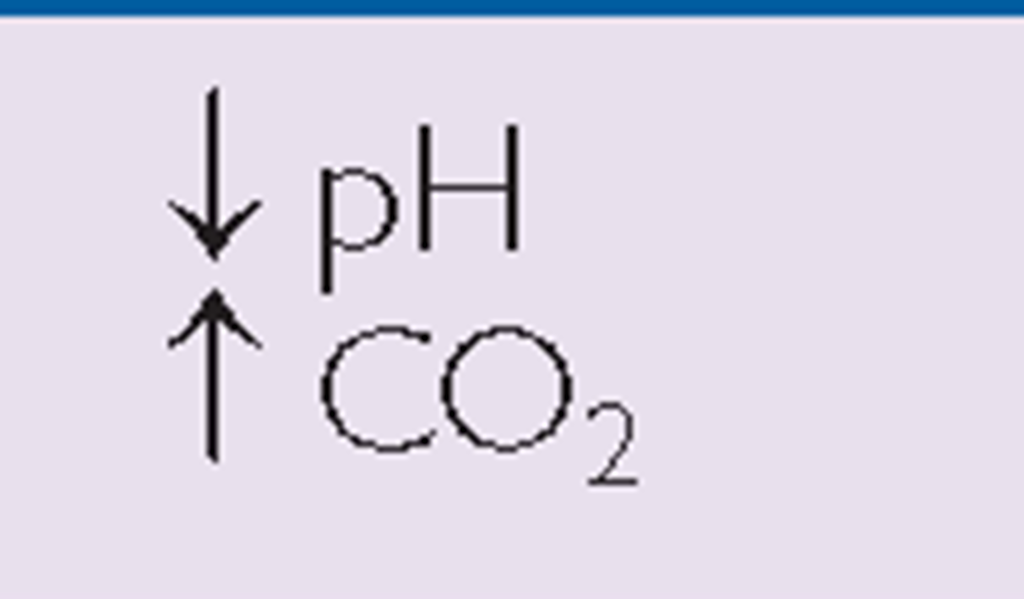
Respiratory acidosis occurs when breathing is inadequate and PaCO2 (respiratory acid) builds up. The extra CO2 combines with water to form carbonic acid, causing a state of acidosis. This is common in emphysema. Respiratory alkalosis (low blood PaCO2) can occur as a result of hyperventilation or excess aspirin intake.
In metabolic acidosis, normal metabolism is impaired, causing a decrease in bicarbonates and a buildup of lactic acid. This can occur in diarrhea, ketosis, and kidney disorders. Metabolic alkalosis occurs when bicarbonate ion concentration increases, causing an elevation in blood pH. This can occur in excessive vomiting, dehydration, and endocrine disorders.
To make blood gas values easier to remember, it is common to use the pH range of 7.35 to 7.45 and a PaCO2 range of 35 to 45 as an approximate guideline for students.
TABLE 17-6. A Comparison of Respiratory and Metabolic Acidosis and Alkalosis
|
ACIDOSIS |
|
|
|
ALKALOSIS |
|
Respiratory |
 |
Normal serum pH |
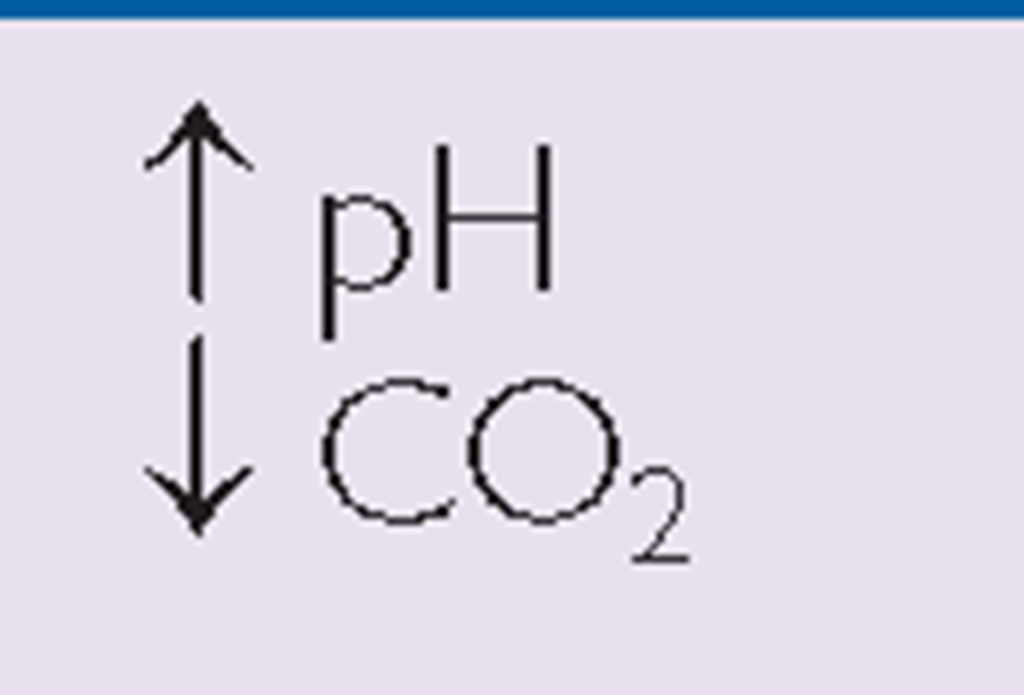 |
Respiratory |
|
Metabolic |
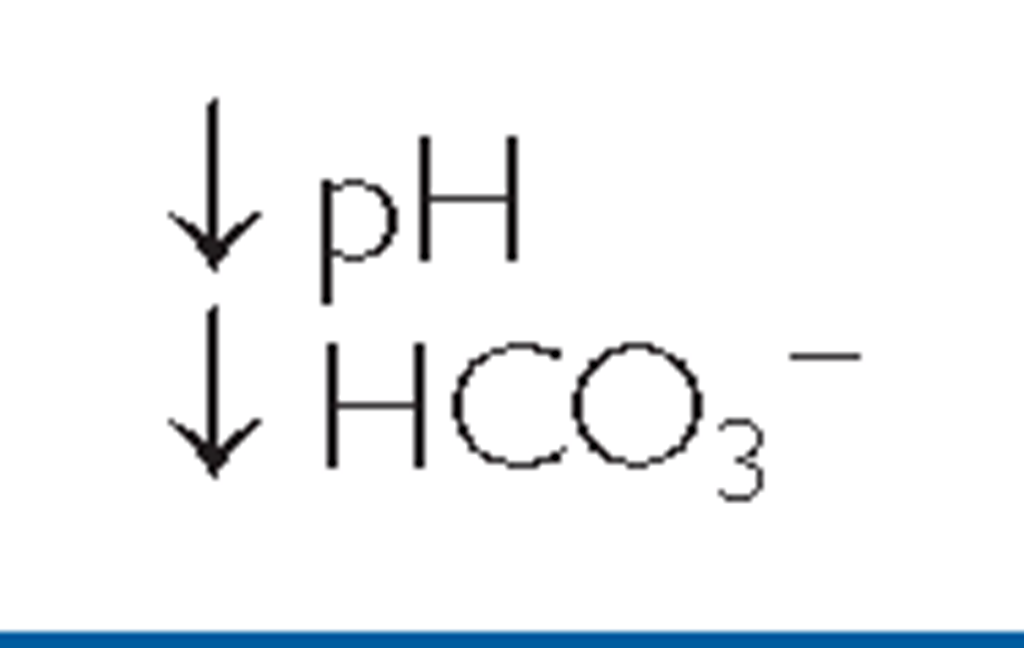 |
7.35-7.45 |
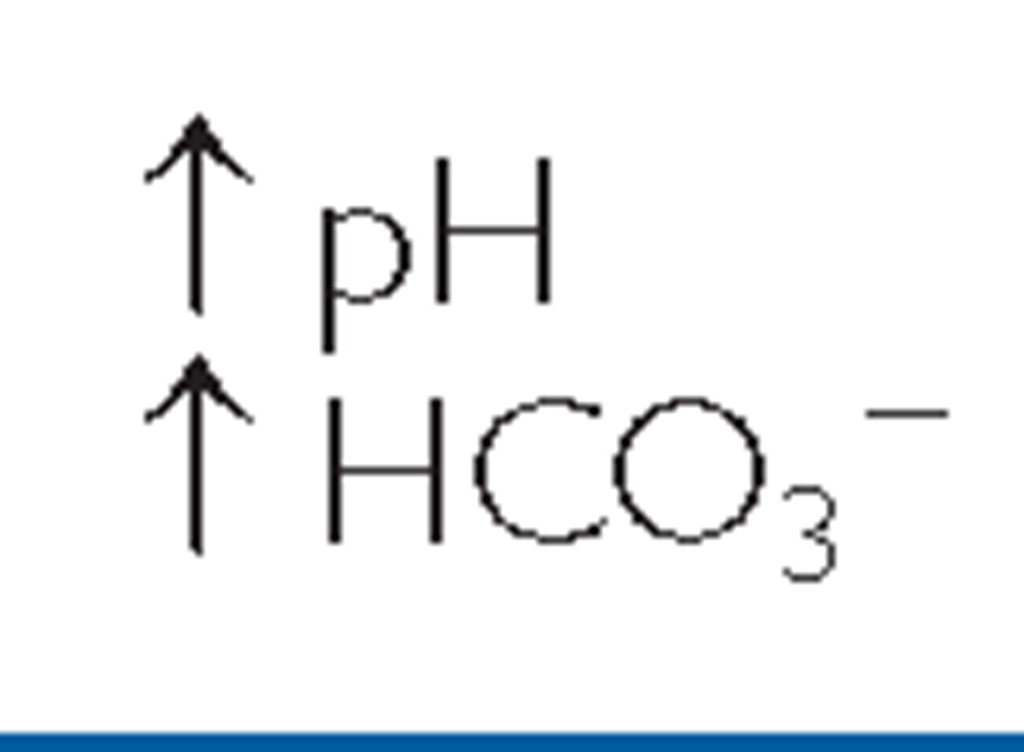 |
Metabolic |
Note: The primary difference between these respiratory conditions is a variation in pH that is influenced by changes in CO2 levels. The primary difference between metabolic acidosis and alkalosis is a variation in pH that is influenced by changes in bicarbonate ion concentration (HCO3_).
Key Concept The pH of the ECF must be maintained between 7.35 and 7.45 for health. Extremes (below 6.8 or above 7.8) are usually fatal. It is important to remember that abnormal levels of blood gases indicate serious problems; these levels are not the causes of the problems. Buffer systems can bind or release H+ in solution, to maintain a constant pH. The carbonic acid/bicarbonate system is the most important extracellular buffer system in the body Other substances that can act as buffers include certain proteins and phosphates.