Potassium
Even though potassium (K+) is the major electrolyte in the ICF, the portion of potassium located in the ECF is important for neuromuscular function, especially cardiac function. Excretion of potassium is done primarily by the kidneys (80%). Potassium is also lost through the bowel (15%) and sweat (5%). Potassium cannot be stored and must be taken in daily. Many different types of drug therapy are associated with potassium imbalances.
Hyperkalemia, a serum potassium level of 5.5 mEq/L or greater, can result from inadequate excretion (e.g., renal failure). However, sustained hyperkalemia is not likely for the individual with normal renal function. Shifts of potassium out of body cells that result from acidosis (increased hydrogen ion concentration in the blood), tissue damage, or burns, can lead to hyperkalemia. Rapid IV infusion of potassium solutions, excessive intake of salt substitutes, or decreased production of aldosterone are also common causes of hyperkalemia. Because stored blood cells gradually release potassium, transfusion of aged blood can also result in hyperkalemia.
Hypokalemia, a serum potassium level of less than 3.5 mEq/L, can result from decreased potassium intake, possibly associated with starvation and alcoholism. Excessive excretion of potassium can occur in renal disease, vomiting, diarrhea, gastric suctioning, excess sweating, diuretic use, or endocrine disorders. Alkalosis can lead to intracellular shifts of potassium, leading to serum potassium deficits. Hypokalemia also contributes to hyperglycemia because of potassium’s effect on both insulin release and organ sensitivity to insulin.
When there is an imbalance of potassium, either hypokalemia or hyperkalemia, the client is at risk for cardiac dysrhythmias. Monitor the cardiac status of the client closely. Check for irregular pulse rhythms and report these to the physician. Carry out orders for electrocardiograms (ECGs) and/or telemetry as ordered.
Calcium
Calcium (Ca++), by exerting a relaxing effect on nerve cells, plays a major role in nerve impulse transmission and muscle contraction. Calcium is also involved in hormone secretion and blood clotting. Hormonal control of the calcium level is achieved by parathormone (from the parathyroid gland) and calcitonin (from the thyroid gland). Calcitriol, the active form of vitamin D, is a regulator of calcium metabolism.
Hypercalcemia, a total serum calcium level of more than 10.5 mg/dL (5.5 mEq/L), most commonly results from cancer or primary hyperparathyroidism. However, immobilization, which promotes bone resorption (decalcification), other endocrine disorders, medications, and abnormal vitamin D metabolism, could cause hypercalcemia.
Although rare, hypocalcemia, a total serum calcium level of less than 9 mg/dL (4.5 mEq/L) or ionized calcium less than 4.6 mg/dL, is most commonly caused by a parathyroid hormone deficit, owing either to surgical or primary hypoparathyroidism or abnormal vitamin D metabolism. In many instances, alkalosis causes a decrease in ionized serum calcium. Other conditions—such as hypoalbumine-mia, hyperphosphatemia, hypomagnesemia, cancer, acute pancreatitis, malabsorption, chronic alcoholism, and some drugs—cause hypocalcemia.
Magnesium
The balance of magnesium (Mg++) depends on normal intake, absorption, and renal excretion. Intracellular and extracellular magnesium concentrations are significant for many important cellular processes, including enzyme reactions, neuromuscular transmission, and cardiovascular tone. Mg++ is closely related to calcium, phosphorus, and potassium.
Hypermagnesemia, a serum magnesium level of greater than 2.5 mEq/L (3.0 mg/dL), is seen in clients with decreased renal excretion owing to diminished renal function. Clients with normal renal function but who have been aggressively treated with over-the-counter medications (antacids and laxatives) or prescribed doses of magnesium may develop hypermagnesemia. Calcium gluconate is the specific antidote for magnesium intoxication.
Hypomagnesemia, a total serum magnesium level of less than 1.5 mEq/L (1.8 mg/dL) is most commonly caused by chronic alcoholism and severe congestive heart failure (CHF) that is being aggressively treated with diuretic therapy. Decreased magnesium intake, malabsorption, and GI losses, including suctioning, vomiting, diarrhea, and fistulas, result in hypomagnesemia. Renal or endocrine diseases, drugs, burns, or shifts of magnesium into cells or bone may also result in hypomagnesemia.
Chloride
Chloride (Cl–) plays a key role in acid–base balance. An excess is called hyperchloremia; a deficit is called hypochloremia. Both of these conditions are associated with acid–base imbalance.
Phosphorus/Phosphate
Phosphorus (P) is a critical component of all tissues. In the human body, phosphorus is often found in the form of phosphate (PO4 –). More than 70% of phosphorus is found in combination with calcium in bones and teeth. To absorb and metabolize phosphorus, vitamin D is needed.
Phosphorus, an important intracellular messenger, is critical for energy production in the form of adenosine triphosphate (ATP). Glycogen needs phosphorus to convert glycogen to glucose. An efficient intestinal tract and normal renal conservation mechanisms are crucial to the normal level of phosphate.
Hyperphosphatemia, an elevation of serum phosphate above 4.5 mg/dL, is commonly caused by decreased renal excretion; redistribution from the ICF to the ECF; or increased intake or intestinal absorption of phosphate. Clients with metabolic acidosis, such as those with renal failure, will often have more ionized calcium present and may exhibit no symptoms of hypocalcemia.
Hypophosphatemia, a serum phosphate of less than 2.5 mg/dL, is unusual but often occurs with respiratory alkalosis owing to prolonged hyperventilation and extensive burn injury. Decreased oral intake or absorption from the GI tract, a shift of phosphate from ECF to ICF, or a loss of phosphate owing to hyperparathyroidism or renal tubule disorder are other causes. Because only 1% of phosphate is in the ECF, decreased serum levels do not necessarily reflect low total body phosphate.
MAINTENANCE OF ACID–BASE BALANCE
The body must maintain acid–base balance to carry out its functions adequately. The body’s cellular activity requires a slightly alkaline medium. ECF is normally maintained at a pH of approximately 7.4, or between 7.35 and 7.45. ICF has a slightly lower pH. Alterations of even a few tenths can be incompatible with cellular activity. An overview of the causes and symptom of acidosis and alkalosis is presented in Table 76-2.
Acidosis
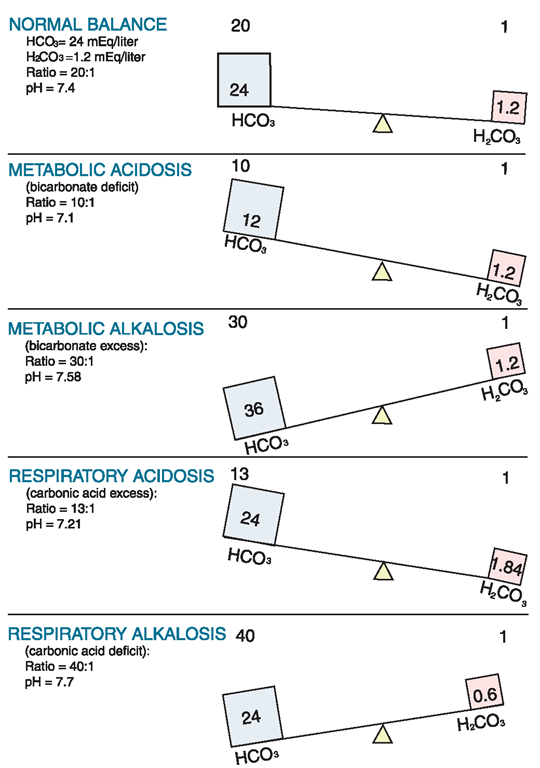
When the blood is more acidic than normal, a state of acidosis exists. A deficit in bicarbonate ions (HCO3 +) or an excess in hydrogen ions (H+) causes a condition called metabolic acidosis. Excess loss of bicarbonate or excessive retention of hydrogen ions owing to renal disease is a common cause. Figure 76-3 illustrates normal and abnormal pH balance (hydrogen ion or H) using bicarbonate ion (H2CO3) and carbonic acid (H2CO3).
An increase in carbon dioxide in the blood characterizes a condition called respiratory acidosis. It may occur in pneumonia, emphysema, and asthma, and after administration of large doses of certain drugs (barbiturates, narcotic analgesics), all of which cause hypoventilation.
TABLE 76-2. Causes and Symptoms of Acidosis and Alkalosis
|
ACID-BASE IMBALANCE |
POSSIBLE CAUSES |
SIGNS AND SYMPTOMS |
|
Metabolic acidosis |
Uncontrolled diabetes mellitus, fasting and starvation (anorexia and bulimia), lactic acidosis, salicylate poisoning (aspirin overdose), alcoholic ketoacidosis, kidney dysfunction and failure, and loss of intestinal secretions (diarrhea, intestinal suctioning, fistulas) |
Decreased pH, decreased HCO3-, diarrhea, nausea and vomiting, anorexia, weakness, lethargy, malaise, altered mental status, coma, peripheral vasodilation, shock, bradycardia, cardiac dysrhythmias, and warm and flushed skin |
|
Respiratory acidosis |
Respiratory center depression (sedative overdose, head trauma); lung disorders, such as pneumonia, emphysema, asthma, pulmonary edema; respiratory distress syndrome; and airway obstruction owing to airway or injury to the thorax, extreme obesity, respiratory muscle paralysis, and kyphoscoliosis |
Decreased pH, increased PCO2, hypoventilation, and shallow respirations; headache, weakness, altered mental and behavioral changes (disorientation, confusion, depression, paranoia, hallucinations), tremors, paralysis, stupor, and coma; warm, dry skin; drowsiness; nausea and vomiting; diarrhea; fruity-smelling breath; acidic blood; and acidic urine |
|
Metabolic alkalosis |
Excess ingestion or administration of sodium bicarbonate, total parenteral nutrition (TPN) solutions containing acetate, parenteral solutions containing lactate, or blood transfusions containing citrate; gastrointestinal (GI) loss of hydrogen ions via vomiting, gastric suction, bulimia, diuretic therapy, and loss of chloride and body fluids |
Increased pH, increased HCO3-, confusion, hyperactive reflexes, tetany, convulsions, hypotension, and dysrhythmias |
|
Respiratory alkalosis |
Hysteria, hyperventilation, high fever, salicylate (aspirin) poisoning, elevated blood ammonia levels, encephalitis, and mechanical ventilation |
Increased pH, decreased PCO2, accompanied by deep respirations with rapid breathing; irritability, panic, lightheadedness, dizziness, paresthesia, positive Chvostek’s and Trousseau’s signs, seizures; nausea, vomiting, and diarrhea; muscle twitching, tetany, and tremors; alkaline urine; and electrocardiogram (ECG) changes |
FIGURE 76-3 · The pH of fluid is balanced using bicarbonate ion (HCO3-), carbonic acid (H2CO3), and hydrogen ions (H+). This figure illustrates pH changes and the balance between HCO3 and H2CO3.
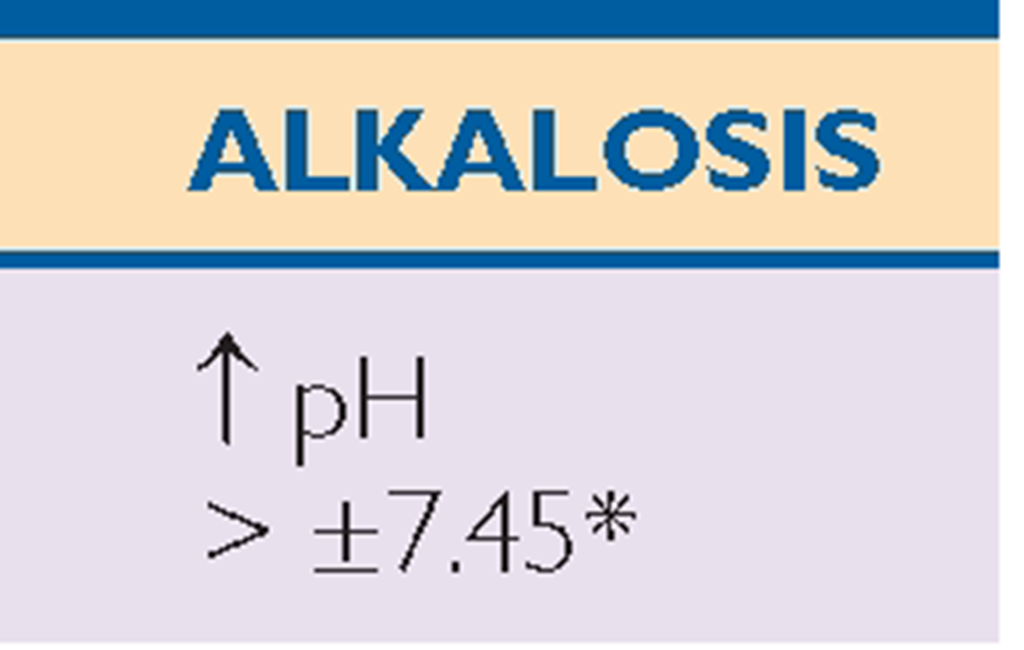
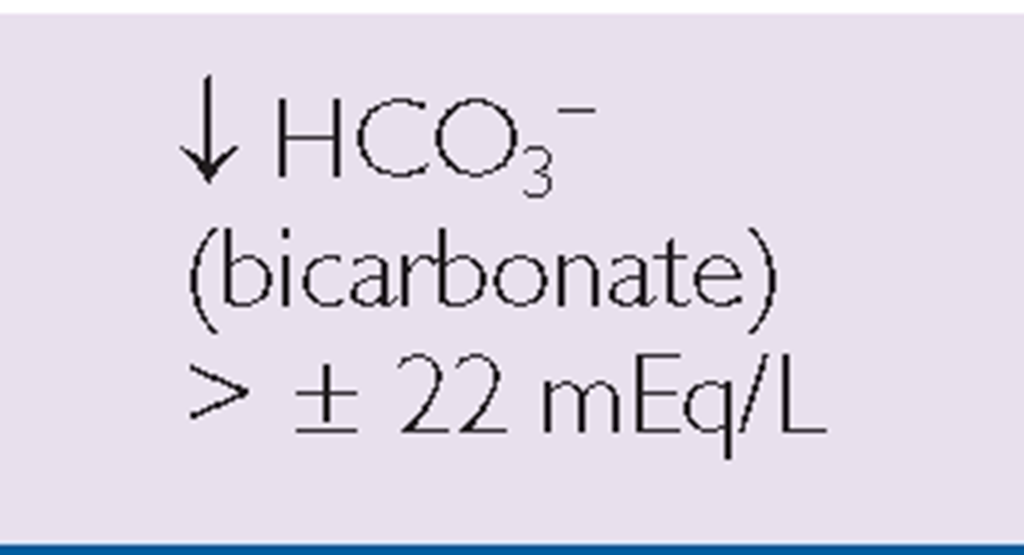
Key Concept ABGs are common diagnostic tests frequently drawn by the respiratory therapist or an RN. Table 76-3 provides a general view of the major aspects of respiratory or metabolic acidosis or alkalosis using the main components of pH, CO2, and bicarbonate (HCO3_).
TABLE 76-3. Clues to Respiratory or Metabolic Acid-Base Balance
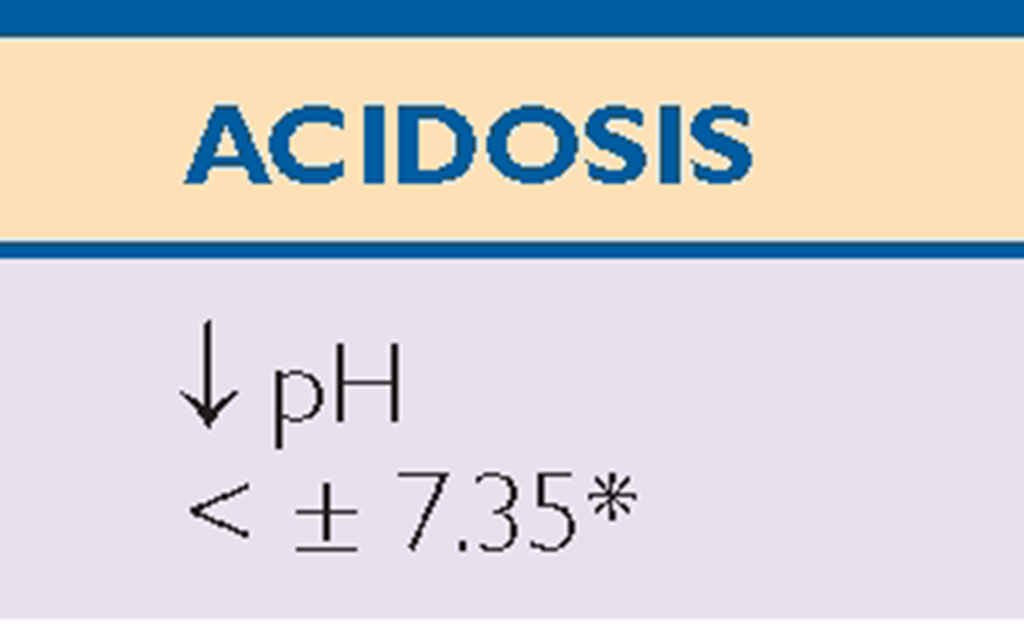 |
 |
|
|
RESPIRATORY |
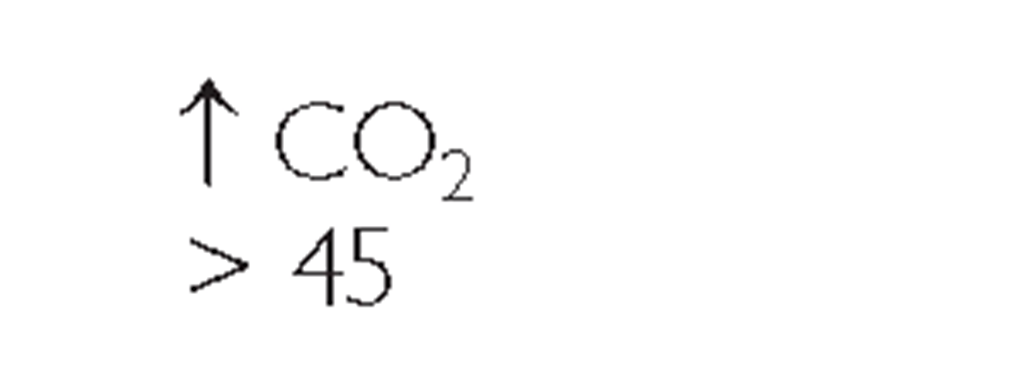 |
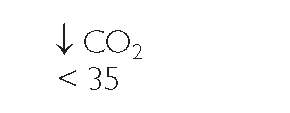 |
|
METABOLIC |
 |
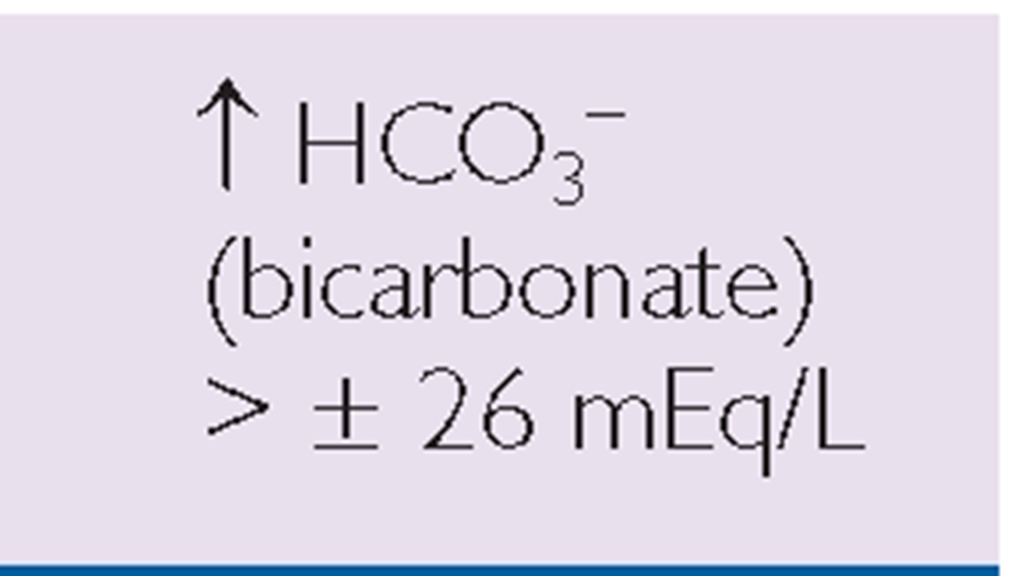 |
*Note: Read symbols in the table as approximately or about 7.35, because there is a great deal of variability in actual levels.
The treatment of choice is correction of the underlying condition. Administer IV infusions, as ordered. The clinician may order bicarbonate for severe metabolic acidosis; however, it should not be given for respiratory acidosis. Lactated Ringer’s solution may be ordered.
The character of respirations and the respiratory rate can indicate changes. Notify the healthcare provider of respiratory changes. The person can be compensating for the condition, or the situation may be worsening. The client may need stimulants or narcotic antagonists to reverse respiratory depression caused by medications. Nursing interventions include placing the client in a position that promotes breathing, such as elevating the head of the bed, or placing the client in the orthopneic position, and administering ordered oxygen. The client may need a mechanical ventilator for severe respiratory acidosis.
Carefully document changes in vital signs. The acidosis may worsen or the client may become alkalotic as an overreaction to the treatment. Monitor laboratory values carefully. Observe the person’s level of consciousness because any change may be significant. The client can lose consciousness if acidosis worsens. The pH of urine can be an indicator of serum acidosis or alkalosis. Acidosis can cause electrolytes to shift, leading to electrolyte imbalances. For example, potassium ions may shift out of the cells in response to metabolic acidosis, resulting in hyperkalemia.
Alkalosis
When the blood is more basic than normal, because of a loss of body acids or excessive retention of alkaline substances, a state called alkalosis exists. Metabolic alkalosis is caused by an excess of bicarbonate, often owing to excess bicarbonate antacid administration or a loss of acids, such as through vomiting or excessive gastric suctioning.
A condition called respiratory alkalosis is a deficit of plasma CO2 (carbonic acid [H2CO3]). This situation is usually caused by hyperventilation. Symptoms are similar to those of metabolic alkalosis. Direct treatment includes reducing the cause of the hyperventilation. Interventions may involve the client using a rebreathing mask or breathing into a paper bag so that he or she will rebreathe his or her own CO2, thus replacing the CO2 needed by the body.
Alkalosis can result in shifts in electrolytes and subsequent electrolyte imbalances. Potassium ions may shift into cells as hydrogen ions shift out of cells in response to metabolic alkalosis. Calcium ions are deionized in states of alkalosis, resulting in symptoms of hypocalcemia. In cases of respiratory alkalosis, serum phosphate may move into the cells, resulting in hypophosphatemia.
Attention to detail is vital. Monitor laboratory values carefully. Administer IV solutions, as ordered, to treat any electrolyte imbalances. Treatment of choice for GI causes is often sodium chloride or potassium chloride, because these electrolytes are usually lost with hydrochloric acid in vomiting. Make sure the alkalosis does not worsen or result in the opposite imbalance. Paresthesia (tingling in the fingers and toes) and muscle twitching are ominous signs of electrolyte loss—particularly the loss of ionized calcium.
KEY POINTS
• Fluid and electrolyte disturbances can occur in anyone, but they are commonly seen in ill and hospitalized clients, including those undergoing surgical and diagnostic procedures. The risk of serious disturbances increases in clients who are at the extremes of the age spectrum.
• Measurement of I&O and daily weight is an important component in the determination of fluid balance.
• Edema is a symptom of many disorders, but most commonly indicates fluid overload. Edematous skin is very friable and susceptible to breakdown. Good skin care is imperative, as is client positioning.
• Electrolyte imbalances commonly involve either an excess or a deficit of the electrolyte. In certain cases, more than one electrolyte imbalance may be occurring.
• The body’s cellular activity requires a slightly alkaline medium. ECF is normally maintained at a pH of approximately 7.4, or between 7.35 and 7.45. ICF has a slightly lower pH. Alterations of even a few tenths can be incompatible with cellular activity.
• Respiratory acidosis, if not corrected, could lead to the need for mechanical ventilation.
• A simple treatment for respiratory alkalosis, usually caused by hyperventilation, is for the client to breathe into a paper bag, thereby retaining needed CO2 in the body.