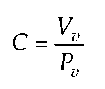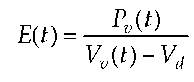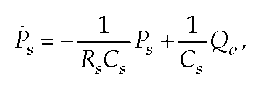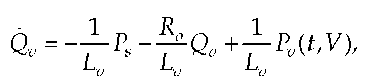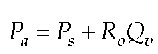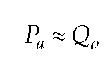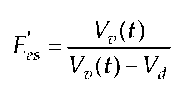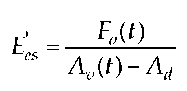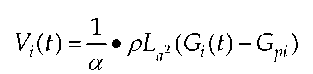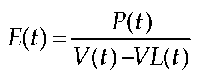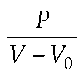Introduction
The present compilation of scientific publications deals with the evaluation of a parameter of myocardial contractility (E’es); the parameter can be exclusively measured using ultrasound, i.e. noninvasive and can be used for a load-independent quantification of myocardial contractility.
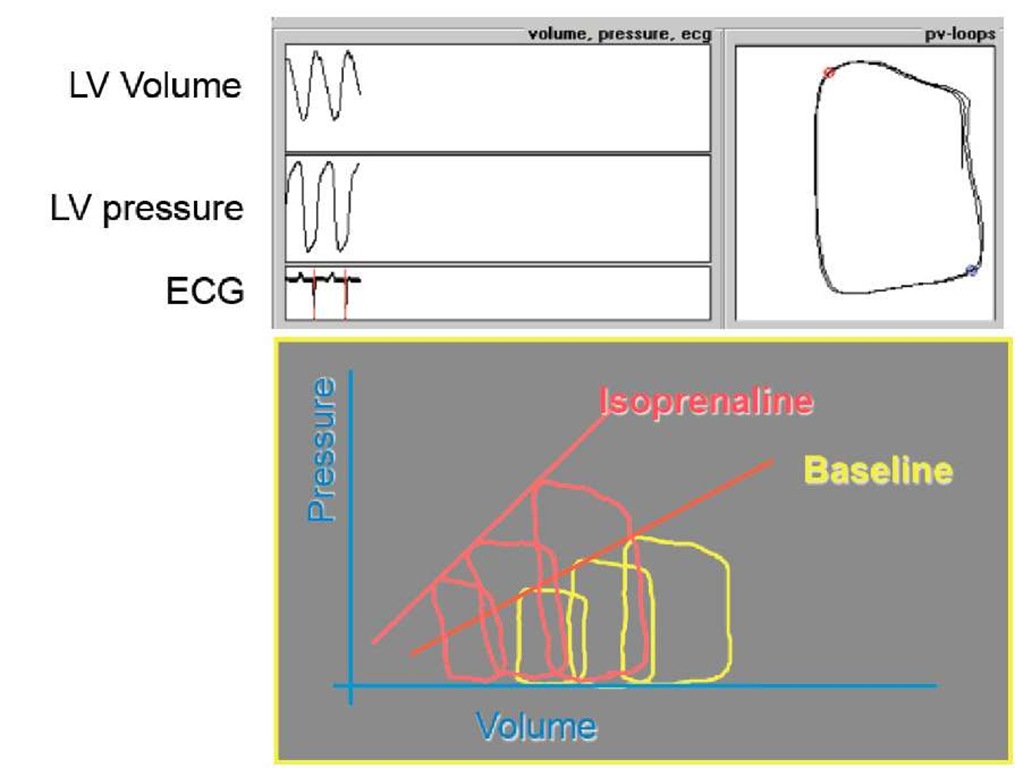
The standard procedure for measuring left ventricular (LV) contractility is by use of a conductance catheter with which LV volume and LV pressure can be determined simultaneously. In 1973, Sagawa (1) was the first to use this catheter, which had been developed at the University Hospital Leiden, and he was able to show that an elastance curve can be determined from a series of loop diagrams which were obtained under acute preload reduction. The slope of the elastance curve (Ees) increased when the positive inotropic substance isoprenaline was administered (Fig. 1).
For clinical use, however, the catheter is a monitoring procedure which is too invasive and thus involves too many risks; in the past few years there have been considerations to replace this procedure with one that is less invasive.
In 1994, Gorscan (2) presented a study with which he was able to show that LV volume can be estimated by continuous echocardiographic determination of the cross-sectional LV area and in 1997 Deanault from the same working group (3) showed that LV pressure can be approximated with the pressure from the radial artery. They generated a loop diagram from these surrogate parameters with which they could estimate LV contractility. In the years 2001-2003 the mathematicians Danielsen, Ottesen and Paladino (4-6) from the University of Roskilde published a model equation from which we deduce that, with simplified assumptions, the Doppler sonographic arterial flow would have to change in proportion to pressure and we asked ourselves if contractility could also be estimated with a flow-area relationship.
Question
The thoughts presented here are a model for measuring LV contractility as it could be determined in the patient, i.e. exclusively based on ultrasound and thus not very invasive.
Fig. 1. Determination of the properties of elastance curves
This raises the question of whether contractility can in fact be estimated using the index E es, which has been deduced from this theoretical model.
If the elastance of the flow-area relationship is exclusively considered as a surrogate parameter to estimate myocardial contractility in the classical sense, then E’es is not a new parameter. If it is assumed, however, that the elastance of the flow-area relationship is based on a completely new mathematical model, i.e. that of the mathematicians Danielsen, Ottesen and Paladino, then we are possibly dealing with a new parameter and deviations between the classical parameter Ees and E’es are then starting points for new examinations.
Background
Ultrasound-based determination of myocardial contractility: theoretical principles
The ability to actively shorten the cardiac muscle, i.e. of contractility, of the left ventricle is based on a complex process (7,8) during which individual mechanisms have to follow one another precisely. Muscle contraction is based on mechanisms and interactions associated with muscle proteins, enzymes, ions and energy sources. The large-scale structure, as well as the small-scale structure of the muscle, are involved in the contractility of the hollow muscular organ which forms the left chamber of the heart or left ventricle. Apart from power transmission towards contraction, the prevention or diversion of shearing forces is an important task which the muscle carries out through mechanisms of mechanical connections, but also through mechanisms of regulation by synchronizing the contraction of the muscle fibers.
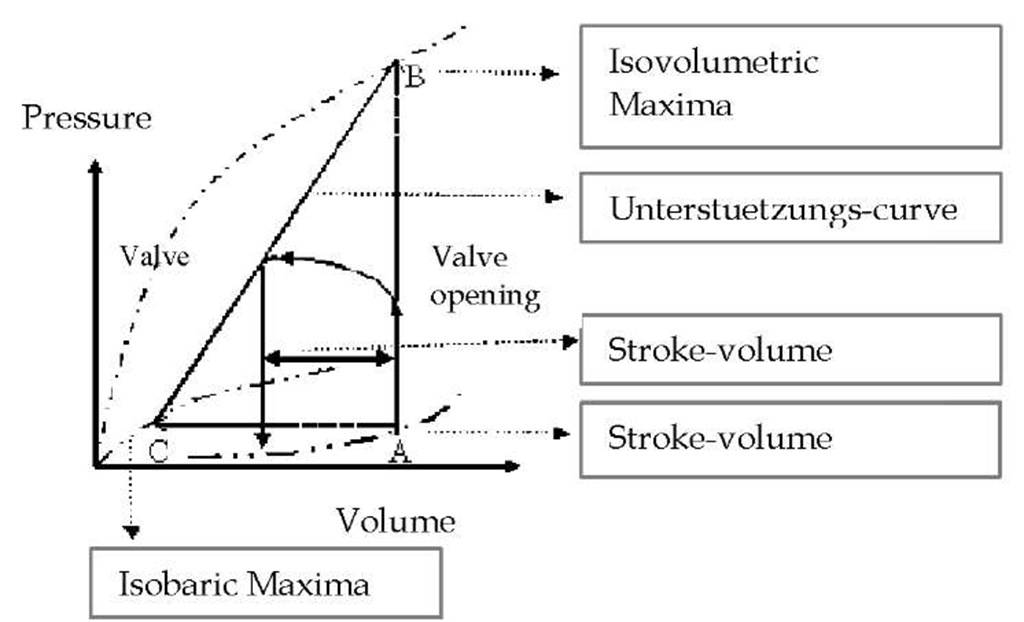
First attempts to describe the contractility of a hollow muscular organ from a mechanical point of view date back to Otto Frank in 1985 (9). Frank determined the pressure of the individual end-diastolic volumes by conducting experiments with a single chamber frog heart as well as the maximum pressure recruitable from this relaxation period (isovolumeric maximum) and the maximum ejection fraction (isotonic or isobaric maximum). The curves established from this resulted in a pressure-volume diagram in which the maximums to be achieved from the relaxation period were connected through the contraction curve (2). Otto Frank deduced the cardiac cycle from this:
Fig. 2. LV pressure-volume diagram by Otto Frank.
The ventricle is filled with volume (preload) and during the process internal pressure in the chamber increases. Then the pressure in the chamber increases through contraction along the isometric maximum against the closed valve until the valve opens which, during the relaxation phase (diastole), prevents reflux from the aorta into the ventricle. While the heart is pumping out volume against the arterial load (afterload), it is pumping in the sense of an auxotonic contraction; this leads to a further increase of arterial pressure and to a decrease in volume through ejection in the ventricle. During the following relaxation phase the pressure in the ventricle decreases to reach its resting level and the chamber is filled again. The cardiac cycle can thus be described through a pressure-volume relationship which is known as the "work diagram" in literature.
In later experiments, Starling (10) deduced the s-shaped connection between the surface of the work diagram and the necessary preload. The two main differences between the concept described by Otto Frank and the one developed by Starling are that the contraction of the left ventricle cannot only be described as a function of filling or preload, but also as a function of time. Another difference is that when it comes to measurements, the left ventricular function is always determined in connection with the arterial vascular system behind it.
In 1959, Warner deduced the calculation of ventricular compliance C from the correlation described by Frank by relating volume Vv and the corresponding pressure Pv at the end of diastole and at the end of systole
and thus created a constant. From this formal approach, two different mathematical terms for the description of LV contraction can be concluded. In 1961, Seelen (11) described contraction through fixed capacitive reactance in series with pressure varying depending on time and in 1973, Suga (1) defined ventricular elastance E(t) through an inverse function of the compliance C(t) function.
with the Si-unit [m_4kgs_2], while Vd represents constant, diastolic volume. The index (t) in this case means that the end-systolic point was determined using an iterative method in which the same loop is crossed by a tangent several times and with varying time (time-varying elastance). In the experimental determination of E(t), the same problems arise as in the correlation already developed by Starling. The index E(t) cannot be determined separately from the vascular system and it is not a function of time.
To get around these problems, Beneken 1965 (12) developed a closed-loop model of the cardiovascular system, which was based on the work of Liljestrand 1938 (13) and Hill 1922 (14), and in which contraction of the heart is calculated through the contractile element described by Hill, embedded in a three-element windkessel model. Further geometrical adjustments yielded an n-element model, which was mathematically described with the finite-element method.
Based on the evaluation described by Hill, Danielsen (4) later developed a model which differentiated between an isovolumetric ventricle and an ejecting left ventricle under arterial load. In the latter model, the direct proportional correlation between arterial pressure Pa and LV outflow Qv was deduced as follows:
with Rs as a global peripheral resistance, Ro as characteristic aortic impedance, Cs as total arterial compliance, Ps as systolic pressure and Ps as the change of arterial pressure with time. This equation can be transformed to This equation can be transformed to:
with Lv as inductance directly above the aortic valve and Qv as a change of flow with time. With the simplified assumption that Lv is constant, the above equation can be simplified as follows.
With the assumption that R0 is constant and Pa changes in direct proportion to Ps the equation is:
Olufsen (15) showed in his one-dimensional dynamic model of the systemic arteries that, corresponding to the Navier-Stokes equation, pressure changes directly proportional to flow in large vessels such as the arteria carotis communis. The model-based experimental examinations confirm that the flow generated through the left ventricle is the connecting link between the heart and vessel blood system.
Against this backdrop, our work group had the idea of developing an index E’es,, analogous to E(t), based on the iteratively determined end-systolic points on the work diagram. This index E’es was to be determined by Vd(t) and blood flow Vv(t) of an artery in proximity to the heart:
with the SI unit [s-1]. The ventricular volume, measured during several cardiac cycles, can be determined using ultrasound technology by estimating it with the help of the surface A in the transgastral short-axis-view. Blood flow Q can be estimated through the blood flow velocity F in the arteria carotis communis measured with the Doppler technology. This then yields the following equation:
The deduced index E’es displays the following important differences compared to the index Ees :
1. By implementing blood flow velocity, the index becomes a function of time.
2. By measuring blood flow velocity in a large artery, the index becomes connected to the vascular system.
3. Through the measuring method used, the index E’es can be completely determined in a noninvasive way, as both F and A are ultrasound parameters.
Measuring methods used
The measuring methods used in this context are conductance and ultrasound methods. Using these methods, myocardial contractility was measured.
Conductance catheter method
A conductance catheter was used for the simultaneous recording of LV pressure and the volume signal (16). The catheter consists of pressure-conductivity transducers. While the blood pressure signal was mechanically measured at the tip of the catheter using a pressure transducer and was subsequently transformed into electric impulses, determination of volume is carried out electromagnetically by measuring the conductivity of the surrounding compartments. The catheter consists of four electrodes and one pressure sensor. The electrodes are fitted below the pressure sensor in pairs. The measurement of cardiac blood volumes using these electrodes is based on an electric field which is established through the outer electrodes in the left ventricle. Two potential differences at the inner electrodes are continuously recorded to measure the conductance between the electrodes in the ventricle.
With the formula
intraventricular volume V(t) is calculated. With a being a volume calibration factor, p being the electric resistance of blood, L being the distance between the electrodes, Gi describing the overall conductance and Gpi describing the conductance of the surrounding tissue; this conductance is called "parallel conductance". This calculation makes a continuous and in vivo recording of volumes in real-time possible.
Ultrasound method
Using the ultrasound-Doppler method, the flow velocity of erythrocytes can be measured with the help of the Doppler effect. The Doppler effect arises because echoes reflected by the erythrocytes have higher or lower frequencies than the signals sent out, depending on the flow velocity and flow direction. The differences in frequency are implemented electronically in readable and recordable curves. In the continuous-wave Doppler (CW Doppler) technique, which is used for detection, one transmitter and one receiver work simultaneously and continuously in the transducer. By mixing it with suitable high frequency signals and with electronic filters, the spectrum of the Doppler frequencies or velocities and the direction can be determined from the returning wave in the evaluation electronics. The disadvantage of this method is that it is not possible to determine the base range of the Doppler echo; however, even relatively high velocities can be recorded.
With the two-dimensional, light-modulated presentation of the echoes, an immediately visible, animated picture is generated through energy and intensity proportional light points that vary according to brightness and as a result of a scan carried out in two directions and in layers. Tissue structures become visible because the points that are represented in shades of gray in relation to the intensity of the echo – as far as they originate from comparable structures – flow together to form areas and lines. The semi-automatic detection of contours serves to detect endocardial interfaces within the "region of interest" (ROI). Semi-automatic contour tracking is based on a seeded ROI algorithm which combines all adjacent pixels within a user-defined signal intensity area. The selected (seed) pixel is the starting point. In automatic segmentation, a small ROI can be positioned in the area of interest. The signal intensity area important for the growth algorithm is automatically taken from the ROI statistics (minimum to maximum value). The area defined in this way can be corrected interactively. For an ROI correction, the boundaries are activated by a click of the mouse. These boundaries can also be corrected interactively. After having selected the acoustic windows relevant for the approximation of volume – transgastral short-axis-view of the left ventricle – the window setting at an end-diastolic phase image was adjusted to achieve an ideal contrast for the detection of endocardial boundaries. This window setting as well as the curves of the ventricular area presented in the ultrasound image were then checked in all phase images of the cardiac cycle. This setting was used for the following examinations.
Measuring parameters
End-systolic elastance is derived from the end-systolic pressure-volume relation (ESPVR). The elastance model was introduced by Sagawa and Suga. (17). Using isolated rabbit hearts, they made the observation that the end-systolic pressure volume coordinates move along a straight line in the case of acute preload reduction. This could also be observed when acute preload reduction occurred at different levels of dilatation of the left ventricle (Fig. 1) is it a picture legend? A difference is made between two elastances: time-dependent elastance Emax and time-varying elastance Ees.
In this model, Emax describes the slope of the end-systolic linear regression curves. In this context the time-dependent elastance E(t) from the equation
is determined, with P(t) = instantaneous intraventricular pressure; V(t) = instantaneous intraventricular volume; V0 = volume, if P(t) = 0. Ees is elastance at the point in time when E(t) reaches its maximum, i.e. when active contraction is at its maximum. Ees is calculated from the slope of the linear regression curves, which is time-varying and results from the maximum of the points.
The calculation of maximum is repeated several times through fixed point iteration until the slope of the line is constant. Ees is determined if, in case of fluctuations in afterload, the time in which the end-systolic state is reached fluctuates and consequently ESPVR does not exactly meet the angle of the loop diagram and Emax is calculated from points which lie ahead of the end-systolic one. Emax is then steeper than Ees. While Emax is the basis for models concerning the mathematical adjustment of the left ventricular function, Ees is used for the experimental quantification of LV contractility. The calculation formula for the indices Emax and Ees is not presented in a consistent manner in literature, however. The index E’es was determined analogous to the index Ees, with the difference that the flow-volume diagram was the basis for calculation
A positive inotropic effect, induced through dobutamine, can be observed through an increase of Ees, i.e. an increase in the slope of the linear regression curves. The opposite occurs in decreasing inotropy, when it is triggered by a |3- receptor blocker like esmolol (Fig. 1) is it a picture legend?, for instance.
Hemodynamic interventions
The main goal of the studies were the controlled, targeted and pathophysiological interventions with sequential application of dobutamine, esmolol, akrinor, hydroxyethyl starch and sodium nitroprusside, with which the degree of correspondence of the two measuring methods was determined. The properties of these substances, which will be described in the following section, were the reason for them being used.