Cardiac Troponins
Cardiac Troponins (cTn) control the calcium-mediated interaction of actin and myosin. It exists in three isoforms: troponin C, troponin I and troponin T. Troponin C exists in all muscle tissues. cTnI is however, completely specific for the heart. cTnT released in small amounts by skeletal muscles, though clinical assays do not detect skeletal TnT. Both have cytosolic or early releasable and structural pools, with most existing in the structural pool. They are more specific compared to CKMB in detection of infarction and are the preferred biomarker for the diagnosis of acute MI (Class I recommendation from the ACC/ AHA task force on diagnosis of AMI).
|
Characteristics |
Troponin C |
Troponin I |
Troponin T |
|
Weight |
18 KD |
26.5 KD |
39 KD |
|
Function |
Calcium binding subunit |
Actomyosin-ATP-inhibiting subunit |
Anchors troponin complex to the tropomyosin strand |
|
Cardiac Specificity |
None |
Yes |
Yes |
Table 3. Troponin Characteristics. KD: Kilo Dalton
Timing of release
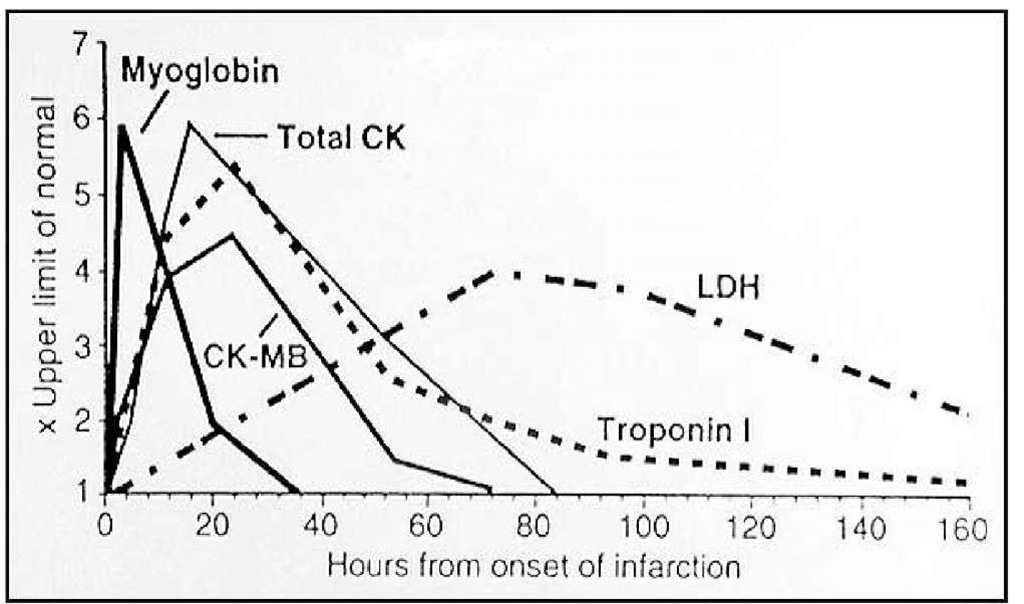
Cardiac troponins begin rising in the blood 4-6 hours post infarction (same time as CKMB). It peaks in 12-24 hours but may take weeks to return to normal. The timing of release, peak and normal return as compared to CKMB has been presented in a graphical form below.
Sensitivity and specificity of cardiac troponins
Cardiac troponins are as sensitive as CK-MB during the first 48 hours after acute myocardial infarction. The sensitivity is 33% from 0-2 hours, 50% from 2-4 hours, 75% from 4-8 hours and approaching 100% from 8 hours after onset of chest pain. The specificity is close to 100%. Troponin elevations have been reported in a variety of clinical scenarios other than acute coronary syndromes. The following is a list of some of the causes for the elevation of troponin in the absence of a thrombotic occlusion of the coronary artery:
• Tachy- or bradyarrhythmias, or heart block
• Critically ill patients, especially with diabetes, respiratory failure or sepsis
• Hypertrophic cardiomyopathy
• Coronary vasospasm
• Acute neurological disease, including stroke or subarachnoid hemorrhage
• Cardiac contusion or other trauma including surgery, ablation, pacing, implantable cardioverter-defibrillator shocks, cardioversion, endomyocardial biopsy, cardiac surgery, following interventional closure of atrial septal defects
• Rhabdomyolysis with cardiac injury
• Congestive heart failure - acute and chronic
• Pulmonary embolism, severe pulmonary hypertension
• Renal failure
• Aortic dissection
• Aortic valve disease
• Apical ballooning syndrome - Takotsubo Cardiomyopathy
• Infiltrative diseases (ie, amyloidosis, hemochromatosis, sarcoidosis, and scleroderma)
• Inflammatory diseases (ie, myocarditis or myocardial extension of endo-/pericarditis, Kawasaki disease)
• Drug toxicity or toxins (ie, adriamycin, 5-flurouracil, herceptin, snake venom)
• Burns, especially if affecting >25 percent of body surface area
• Extreme exertion
• Transplant vasculopathy
The 2007 joint ESC/ ACCF/ AHA/WHF task force recommends that an elevated value of cardiac troponin, in the absence of clinical evidence of ischemia, should prompt a search for other causes of myocardial necrosis as listed above.
Troponin assays
The skeletal and cardiac isoforms of troponin T and troponin I are distinct, and skeletal isoforms are not detected by the monoclonal antibody-based assays currently in use. This specificity for cardiac isoforms is the basis for the clinical utility of cTnT and cTnI assays. Contemporary troponin assays are quite sensitive and can detect very small amounts of myocardial necrosis (<1 g). Troponin C is not used clinically because both cardiac and smooth muscle share troponin C isoforms. The ESC/ ACC recommended that the diagnosis of MI be based on troponin levels in excess of the 99th percentile of a reference control group. As cTnT and cTnI levels are undetectable in most normal subjects, the 99th percentile is very low (eg, 0.04 to 0.5 micrograms/L). However, most assays are imprecise at this low level, and so it has been recommended that the definition of MI be raised to that value at which a specific assay has a coefficient of variation of 10 percent or less. New guidelines embrace 99th percentile for two reasons. This level is also low (0.1 to 1.2 micrograms/L), but higher than the 99th percentile standard. Due to variations in assay precision and individual laboratory policies, the upper limit of normal varies between laboratories, but in all cases is above the 99th percentile.
Point-of-care assays
The National Academy of Clinical Biochemistry (NACB) recommendations specify that cardiac markers be available on an immediate basis 24 h/d, 7 d/wk, with a turnaround time of 1 hour. Point-of-care (POC) devices that provide rapid results should be considered in hospitals whose laboratories cannot meet these guidelines. POC assays for CK-MB, myoglobin, and the cardiac troponins TnI and TnT are available. Only qualitative TnT assays are available as POC tests, but both quantitative and qualitative POC TnI assays are currently marketed. In a multicenter trial, the time to positivity was significantly faster for the POC device than for the local laboratory (2.5 h vs 3.4 h).In another multicenter study, which evaluated the i-STAT POC TnI assay in comparison with the central laboratory in 2000 patients with suspected ACS, POC testing reduced the length of stay by approximately 25 minutes for patients who were discharged from the ED. The sensitivity of current POC assays coupled with the benefit of rapid turnaround time make the POC assays attractive clinical tools in the ED.
Prognostic value of cardiac troponins
In addition to its use in the diagnosis of MI, an elevated troponin level can identify patients at high risk for adverse cardiac events. Specifically, data from a meta-analysis indicated that an elevated troponin level in patients without ST-segment elevation is associated with a nearly 4fold increase in the cardiac mortality rate. In patients without ST-segment elevation who were being considered for thrombolytic therapy, initial TnI levels on admission correlated with mortality at 6 weeks, but CK-MB levels were not predictive of adverse cardiac events and had no prognostic value. Other studies revealed that an elevated troponin level at baseline was an independent predictor of mortality, even in patients with chest pain and acute MI with ST-segment elevation who were eligible for reperfusion therapy. Finally, the TIMI IIIB, GUSTO IIa, GUSTO IV ACS, and FRISC trial all demonstrated a direct correlation between the level of TnI or TnT and the mortality rate and adverse cardiac event rate in ACS.
High Sensitive Troponin (hsTroponin)
High-sensitive assay is one which has a total imprecision of less than 10% at the 99th percentile and some would propose also being able to quantitate over 50% of normal values below that 99th percentile. High-sensitive cTn assays have two differentiating features from contemporary cTn assays: 1) detection of cTn in healthy persons and 2) a precise definition of what is "normal" (= the 99th percentile). Recent multicenter studies have shown that high-sensitive cTn assays improve the early diagnosis of acute myocardial infarction (AMI). To achieve the best clinical use, cTn has to be interpreted as a quantitative variable. Rising and/or falling levels differentiate acute from chronic cardiomyocyte necrosis. The term "troponin-positive" should therefore be avoided. "Detectable" levels will become the norm and have to be clearly differentiated from "elevated" levels. The differential diagnosis of a small amount of cardiomyocyte necrosis and therefore mild elevation of cTn is broad and includes acute and chronic cardiac disorders. The differential diagnosis of a large amount of cardiomyocyte necrosis and therefore substantial elevation of cTn is much smaller and largely restricted to AMI, myocarditis and Takotsubo cardiomyopathy. Two large prospective multicenter studies showed that sensitive and high-sensitive cTn assays have a higher diagnostic accuracy compared to contemporary cTn assays at presentation, in the diagnosis of AMI. Earlier "rule in" may reduce morbidity by allowing earlier revascularization, earlier transfer to the coronary care unit, and earlier initiation of evidence-based AMI treatment. Nonetheless, the improvement in sensitivity is at the expense of specificity. There is still considerable controversy in regard to how to use these assays to detect acute events such as AMI. Hence, at this time it has not been approved for clinical use and is yet in research phase.
Myoglobin
Myoglobin is a heme protein found in skeletal and cardiac muscle that has attracted considerable interest as an early marker of MI. Its low molecular weight accounts for its early release profile: myoglobin typically rises 2-4 hours after onset of infarction, peaks at 612 hours, and returns to normal within 24-36 hours. Rapid myoglobin assays are available, but overall, they have a lack of cardiospecificity. Serial sampling every 1-2 hours can increase the sensitivity and specificity; a rise of 25-40% over 1-2 hours is strongly suggestive of acute MI. However, in most studies, myoglobin only achieved 90% sensitivity for acute MI, so the negative predictive value of myoglobin is not high enough to exclude the diagnosis of acute MI. The original studies that evaluated myoglobin used the WHO definition of acute MI that was based on a CK-MB standard. With the adoption of a troponin standard for acute MI in the ACC/ESC definition, the sensitivity of myoglobin for acute MI is substantially reduced. This significantly diminishes its utility, and a number of studies have indicated that contemporary cardiac troponin assays render the use of myoglobin measurements unnecessary.
|
Conditions where myoglobin increases |
Conditions where myoglobin does not increase |
|
Acute myocardial infarction |
Non cardiac chest pain |
|
Vigorous exercise |
Mild to moderate exercise |
|
Open heart surgery |
CHF without acute myocardial infarction |
|
Rhabdomyolysis |
Cardiac catheterization |
|
Progressive muscular dystrophy |
|
|
Shock |
|
|
Renal Failure |
Table 4. Serum myoglobin in various clinical conditions
Timing of release, peak and return to baseline of various cardiac markers
CAD is highly prevalent in patients with CKD, making interpretation of cardiac markers important. Despite this, interpretation of elevated cardiac enzymes in patients with renal failure is often confusing at best. Elevations in serum troponin often observed in asymptomatic patients with chronic kidney disease. Even using the most conservative cutoff values, a disproportionate number of patients still have elevated troponins. The mechanism for this is unclear. In a 2002 study in Circulation, 733 asymptomatic patients with ESRD were evaluated. Using conservative cutoff values,
- 82% had elevated cTnT
- 6% had elevated cTnI
Of those 733 asymptomatic patients on HD, 2-year mortality rates were 52% in those with cTnI >0.1 ^g/ dL .These data have been corroborated in a number of smaller studies in similar populations. Serial measurements are helpful in the setting of possible ACS. cTnI appears to be much less likely to be associated with false positives in the CKD population than cTnT, making it the preferred biomarker in this setting.
Fig. 4. A comparison of CKMB, cardiac troponins and myoglobin.
Markers of inflammation
Acute coronary syndromes are caused by vulnerable plaques. It is thought that one of the driving forces causing atheromatous plaques to rupture or erode, causing a cascade of events leading to coronary artery occlusion, is inflammation in the plaques. In this section cardiac inflammatory markers are dealt with which is at the verge of entering into clinical practice as tool for diagnosing and predicting future cardiovascular events at earlier stage and for risk stratification. Highly Sensitive Creative Protein (hsCRP), Myeloperoxidase (MPO), Matrix Metalloproteinases (MMP), Pregnancy Associated Protein A(PAPP-A),Placenta Growth Factor(PIGF) are reviewed.
C-Reactive protein (CRP)
C Reactive Protein is an acute phase reactant synthesized in liver and is elevated in inflammatory conditions. Once ligand-bound, CRP can activate the classical compliment pathway, stimulate phagocytosis and bind to immunoglobulin receptors. "High-sensitivity" only means that the concentration of CRP was determined using an assay designed to measure very low levels of CRP. The American Heart Association has defined risk groups as follows: Low risk: less than 1.0 mg/L, Average risk: 1.0 to 3.0 mg/L and High risk: above 3.0 mg/L. Two assays averaged fasting or non fasting, and optimally 2 weeks apart, provide a more stable estimate of level of this marker. If a level is greater than 10 mg/L is identified, there should be a search initiated for an obvious source of infection or inflammation, which could obscure any prediction of coronary risk that might be attributed to the elevated level. The landmark study by Liuzzo et al. showed that patients presenting with unstable angina and elevated plasma concentrations of CRP had a higher rate of death, MI and need for re-vascularisation compared with patients without elevated concentrations. In more recent trials, other investigators have confirmed the increased risk in ACS associated with higher CRP concentrations. In each of the above studies, the predictive value of CRP was independent of, and additive to, cardiac troponin. More importantly, CRP was found to have prognostic value even among patients with negative cardiac troponin and no evidence of myocyte necrosis. Methodological issues have however been highlighted and the independence between CRP and troponin release questioned. Therefore , although many studies have suggested that low-grade hsCRP elevations are independently associated with coronary risk, more complete evidence is needed to validate the use of hs-CRP as a risk assessment tool in general practice and as a target for therapy in individual patients .
Myeloperoxidase (MPO)
Myeloperoxidase (MPO) is a hemoprotein that is abundantly expressed in polymorphonuclear cells (neutrophils) and secreted during their activation. The presence of a peroxidase in the cytoplasmic granules of leukocytes was suggested at the beginning of 20th century but it was the early 1940s that it was purified for the first time. MPO plays an important role in neutrophil microbicidal action through catalyzing chloride ion oxidation to hypochlorous acid, which is a potent antimicrobial agent. On the other hand, it was demonstrated that MPO causes oxidative modification of low density lipoprotein (LDL) to a high uptake form that is considered to be a key event in the promotion of atherogenesis. Hence myeloperoxidase is believed to participate in the initiation and progression of cardiovascular diseases. MPO possesses potent proinflammatory properties and may contribute directly to tissue injury.
In a study consisted of patients diagnosed with ACS and other heart disease or unspecified chest pain, considerably higher MPO concentrations were demonstrated in the troponin-negative ACS patients on admission who became troponin-positive after 6h. This suggests that level of MPO possessed remarkably higher sensitivity than assessment of cTnI alone in all patients with ACS. MPO levels are associated with the presence of angiographically proven coronary atherosclerosis. In addition to clinical history and other tools MPO has been approved by FDA as cardiac biomarker to evaluate the patients with chest pain and at high risk for coronary artery disease.