Pathways to Regions Mediating Motor Functions
We have previously established that the reticular formation receives inputs from two regions that mediate motor functions: the sensorimotor cortex and cerebellum. Inputs from both of these regions modulate the activity of neurons in the reticular formation that project to the spinal cord. Recall that the afferents from both of these regions and, in particular, corticoreticular fibers terminate near the origins of these descending reticulospinal fibers.
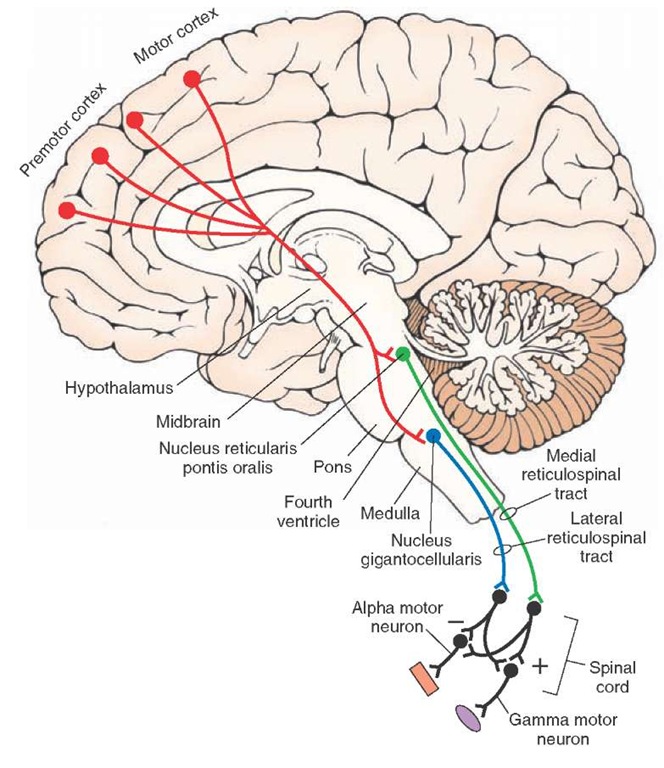
The primary motor outputs of the reticular formation are directed on the spinal cord as reticulospinal fibers.The fibers arising from the pons form the medial reticulospinal tract and issue from the nucleus reticularis pontis oralis and nucleus reticularis pontis caudalis. They pass in the ventral funiculus of the spinal cord and facilitate both alpha and gamma motor neurons of extensors. In contrast, fibers from the medulla arise from the magnocellular nuclei, such as the nucleus gigantocellularis, and descend in the ventral funiculus as the lateral reticulospinal tract, where they also terminate on alpha and gamma motor neurons of extensors. However, their action on these neurons is inhibitory. Collectively, the actions of the reticulospinal fibers serve to modulate muscle tone, regulate posture, and participate in automatic reflex mechanisms involving the extensor musculature.
Alterations in posture and muscle tone can be mediated by the corticoreticular pathways as part of the overall voluntary motor control system.
The actions of the cerebellum are somewhat different. The reticular formation receives feedback signals from the fastigial nucleus as part of an automatic regulatory mechanism. The feedback message to the reticular formation is produced in response to signals that the cerebellar cortex receives from the reticular formation. Thus, the efferent connections of the reticular formation to the cerebellar cortex complete a feedback circuit linking these two important structures for the regulation of posture and muscle tone. The efferent pathways to the cerebellar cortex from the reticular formation arise from two nuclei of the medulla (the lateral and paramedian reticular nuclei) and one nucleus from the pons (reticulotegmental nucleus).
The closely integrated response network between the cerebellum, cerebral cortex, and reticular formation is extremely important for the proper maintenance of postural mechanisms and standing erect, especially because there is a delicate balance between the excitatory and inhibitory actions of different regions of the reticular formation on extensor motor neurons. Disruption of any component of this network can lead to significant motor deficits. These deficits include spasticity if the inhibitory components of the reticulospinal pathways are disrupted by loss of corticoreticular inputs, rigidity if the inputs from the cerebellum to the inhibitory zones of the reticular formation are disrupted, and hypertonicity if cerebellar inputs on the excitatory components are disrupted.
Other motor functions that involve the reticular formation include the control of horizontal eye movements.This region integrates signals from the cerebral cortex (frontal eye fields) and vestibular nuclei. As a result, the horizontal gaze center enables conjugate horizontal movements of the eyes to occur, especially in response to changes in body posture and position of the head in space.
FIGURE 23-13 Descending motor pathways to the spinal cord from the pons and medulla. Note also that corticoreticular fibers from motor and premotor cortices modulate the activity neurons of the reticular formation that give rise to the reticulospinal tracts. In turn, reticulospinal tracts modulate the activity of alpha and gamma motor neurons. Activation of the lateral reticulospinal tract inhibits spinal reflexes (-), and activation of the medial reticulospinal tract facilitates (+) spinal reflexes.
FIGURE 23-14 Effects of stimulation of reticular formation on spinal reflexes. Stimulation of the facil-itory zone ([+] shown in green) of the reticular formation causes a dramatic increase in the patellar reflex as determined by electromyographic (EMG) measurements, whereas marked suppression of this reflex follows stimulation of the inhibitory zone ([-] shown in red) of the reticular formation.
Pathways Mediating Autonomic Functions
Earlier in this topic, we noted that parts of the reticular formation receive autonomic-related inputs from first-order signals associated with changes in levels of blood oxygen and carbon dioxide levels and changes in blood pressure and inputs from higher order regions of the brain that function to regulate blood pressure and heart rate.In brief, the reticular formation of the lower brainstem possesses both excitatory and inhibitory regions that provide neural control over cardiovascular functions. The most important controlling regions include the solitary nucleus and the caudal and rostral ventrolateral medulla for blood pressure because they receive inputs from primary and secondary autonomic afferents as well as from higher regulatory centers of the brain, such as the hypothalamus, midbrain PAG, and amygdala. Recall that the solitary nucleus and ventrolateral medulla share reciprocal connections and that the ventro-lateral medulla can influence cardiovascular functions relatively directly because its descending axons reach the intermediolateral cell column of the thoracic and lumbar cord.
Respiratory mechanisms involve nuclear groups located in both the dorsal and ventrolateral medulla and pons. Descending reticulospinal projections to the cervical cord make synaptic contact with somatic motor neurons of the phrenic nerve that innervate the diaphragm. This provides the efferent pathway by which the reticular formation can modify respiratory responses.
Pathways Modulating Functions of the Hypothalamus and Limbic System
Projections from different regions of the reticular formation (but most notably, the midbrain PAG) and neuronal groups containing monoamine neurons to the hypothala-mus and limbic structures are now well established. These efferent projections probably play significant roles in the regulation of higher-order autonomic, endocrine, and behavioral functions associated with these structures.
The importance of these ascending fibers is demonstrated by two types of experiments. The first is that lesions of the reticular formation disrupt functions associated with the hypothalamus and limbic system. The second is that administration of drugs that block one or more of the monoamine systems can affect mood changes that are normally associated with the hypothalamus and limbic system.
Sleep and Wakefulness
Many regions of the CNS mediate sleep and wakefulness. These regions include specific nuclei of the reticular formation, thalamus, hypothalamus, and basal forebrain. In this section, we will concentrate on the involvement of the reticular formation in sleep and wakefulness.
Stages of Sleep
Most of nighttime sleep (approximately 75%) is not associated with rapid eye movements (REM [Fig. 23-15]). This activity is mediated mainly by thalamocortical circuits. These circuits are ultimately activated by the brainstem. There is an increase in gamma aminobutyric acid (GABA)-ergic neuronal activity in the anterior hypothalamus and a decrease in noradrenergic, serotonergic, and cholinergic activity. The neuronal activity affected by these neurotransmitters is likely mediated on the thalamus and cortex and is linked to non-REM sleep.
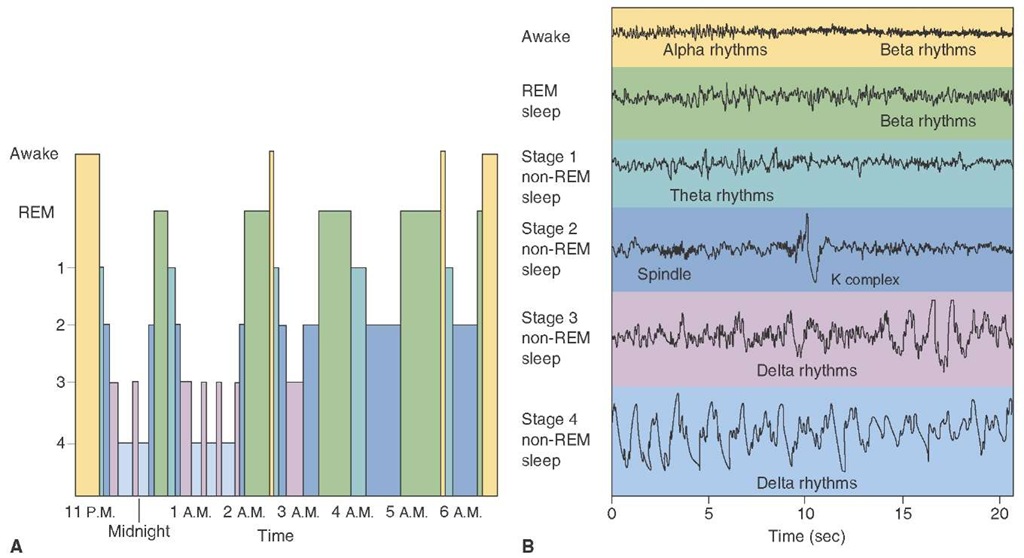
FIGURE 23-15 Electroencephalographs (EEG) rhythms during different stages of sleep. (A) Graph depicts the extent to which different stages of sleep are present throughout the night, beginning at 11:00 p.m. when sleep began. Initially, there were deeper periods of non-REM (rapid eye movement) sleep, which were eventually replaced by longer periods of REM sleep. The sleep cycles tended to be repeated, with the REM sleep becoming more prominent. (B) The four stages of sleep are characterized by the presence of different EEG rhythms. For example, theta rhythms are present during stage 1, sleep spindles are present in stage 2, and delta rhythms are present in stages 3 and 4. Note that, during REM sleep, the EEG displays a beta rhythm, which is characteristic of the waking state.
The initiation of non-REM sleep begins with brain wave (EEG) slowing and vertex waves or high-voltage slow waves recorded from the vertex of the head, called drowsiness or stage 1 sleep. As sleep deepens, stage 2 evolves from stage 1 and is characterized by slowing of the EEG and clusters of 12 to 14 Hz rhythmic waves ("sleep spindles") recorded in the central head region. A combination of sleep spindles and K-complexes are high-voltage, irregular, slow waves also seen during stage 2 sleep. Stage 3 is characterized by high-voltage (75 ||V) slow waves of frequencies in the range of 2 Hz. Sleep is moderate to deep, and spindle activity declines. Stage 4 sleep is characterized by more than 50% slow-wave activity. The circuitry of lighter non-REM sleep consists of rhythmic activity generated by thalamic and cortical neurons. As sleep deepens, cortical neurons generate slow (delta frequency) waves. Stage 4 sleep is the most difficult stage to wake someone from, although sleep walking and talking are recorded from stage 3 or 4 sleep.
Rapid Eye Movement (Paradoxical) Sleep and Its Anatomical Loci
Studies have demonstrated that the pontine reticular formation is necessary for REM or "dreaming" sleep. These studies also demonstrated that the pons was instrumental in the inhibition of muscle tone during REM sleep. Projections from the pontine reticular formation to the spinal cord are essential to prevent the "acting out" of dreams. Ponto-geniculo-occipital waves, as measured in electrical recordings, occur in bursts during REM sleep, originating in the nucleus pontis oralis and projecting to the forebrain. These waves serve as a marker for REM sleep in the cat but have never been demonstrated in humans.
Two pontine reticular nuclei initiate REM sleep. Because of their functional similarity, the pedunculopon-tine nucleus and the lateral dorsal nucleus are commonly considered to be one region. These neurons contain cholinergic nuclei that project to other regions of the reticular formation, hypothalamus, basal forebrain, and thalamus. When the cholinergic regions become activated, there is a change in the activity within the thalamus and cortex. Moreover, as indicated earlier, REM sleep is associated with activation of these cholinergic neurons and a reduction of catecholaminergic activity.
Multiple regions modulate REM sleep. The locus ceru-leus, a major norepinephrine cell group of the rostral pontine reticular formation, projects to the thalamus and cerebral and cerebellar cortices. These connections provide the anatomical bases for the regulation of sensory functions of the cerebral cortex as well as cortical activation itself, which can affect REM sleep. The serotonergic region of the reticular formation, the raphe nuclei, innervates much of the forebrain. It has been hypothesized that serotonin contributes to the process of REM sleep. Consistent with this hypothesis is the observation that destruction or pharmacological depletion of serotonin levels in the brain results in long periods of wakefulness (insomnia). Replacement therapy with 5-hydroxy tryptophan (the serotonin precursor) can restore normal states of slow-wave sleep.
Wakefulness, as well as REM sleep, is modulated by the reticular formation. The direct connections of the monoaminergic fibers to the cerebral cortex endow this region with a simple mechanism by which the reticular formation can regulate levels of cortical excitability. In particular, the noradrenergic neurons located in the nucleus locus ceruleus seem to play an important role in cortical excitability and wakefulness. Sleep onset occurs when circadian rhythms, any sleep deprivations, and environmental stimulation (such as a dark room) are optimal. Removal of the cortical-activating influence occurs, and non-REM sleep is initiated.
In experimental studies, the nucleus locus ceruleus has also been linked to the regulation of REM or paradoxical sleep (called paradoxical because of the EEG pattern characteristic of the awake state). Lesions placed in the locus ceruleus or administration of the drug alpha methyldopa (from which the false neurotransmitter alpha methylnore-pinephrine is synthesized) can suppress paradoxical sleep. Moreover, long-lasting states of wakefulness are produced after cerebral catecholamine levels are elevated.
Role of Other Regions in Sleep and Wakefulness
Although not part of the reticular formation, neurons in the suprachiasmatic nucleus of the hypothalamus also appear to play an important role in the sleep-wakefulness cycle. These neurons show a clear-cut diurnal rhythm for light and darkness. They receive direct retinal inputs, and if the nucleus is destroyed, other rhythms, such as those for endocrine function and sleep-wakefulness cycles, are disrupted. What remains unknown is the specific triggering mechanism for sleep. It had previously been thought that it was located in the raphe complex, but as indicated earlier, more recent studies suggest involvement of the cholin-ergic neurons in the pedunculopontine region. Other investigators further suggest that the suprachiasmatic nucleus serves this function. Whatever mechanism(s) regulate sleep and wakefulness, it is clear that it is an active, not a passive, process that is present, and one that involves the reticular formation and other nuclei in the brainstem and forebrain.
![Effects of stimulation of reticular formation on spinal reflexes. Stimulation of the facil-itory zone ([+] shown in green) of the reticular formation causes a dramatic increase in the patellar reflex as determined by electromyographic (EMG) measurements, whereas marked suppression of this reflex follows stimulation of the inhibitory zone ([-] shown in red) of the reticular formation. Effects of stimulation of reticular formation on spinal reflexes. Stimulation of the facil-itory zone ([+] shown in green) of the reticular formation causes a dramatic increase in the patellar reflex as determined by electromyographic (EMG) measurements, whereas marked suppression of this reflex follows stimulation of the inhibitory zone ([-] shown in red) of the reticular formation.](http://what-when-how.com/wp-content/uploads/2012/04/tmp3625_thumb22_thumb.jpg)