Motor Systems
It is clear that the reticular formation plays an important role in the processing of sensory information. The likely mechanisms are discussed in the following sections. As noted in previous topics concerning motor systems, the reticular formation also plays an important role in the regulation of motor responses. The magnocellular nuclei of the reticular formation of the medulla and pons receive significant inputs from two key regions associated with motor functions: the cerebellum and sensorimotor cortex.
Cerebellum. The reticular formation and cerebellum share reciprocal connections, which complete a circuit comprising feedback pathways between these two regions for the regulation of motor functions associated with each of these two structures. Cerebellar fibers that project to the reticular formation of the medulla and pons arise from the fas-tigial nucleus. The fibers are both crossed and uncrossed and use the uncinate fasciculus (the pathway that passes just dorsal to the superior cerebellar peduncle). Cerebral Cortex. Fibers from the same region of the sensorimotor cortex (areas 4, 3, 1, and 2) that give rise to much of the corticospinal tract also give rise to corticoreticular fibers. Corticoreticular fibers terminate in the pons and medulla near cell groups that give rise to the reticulospi-nal tracts (nucleus reticularis pontis oralis and nucleus reticularis pontis caudalis for the pons and nucleus gigan-tocellularis for the medulla [Fig. 23-5]). In this way, corti-coreticular fibers can influence both voluntary as well as reflex motor functions by acting on those neurons of the reticular formation that control extensor motoneuron activity.
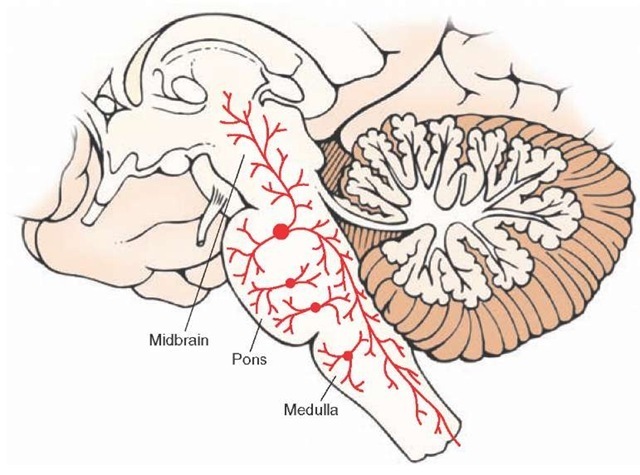
FIGURE 23-5 Corticoreticular projections. Sagittal view of the brain depicting the principal projections of fibers from the cerebral cortex to the reticular formation (shown in red). The largest majority of fibers arise from the motor and premotor cortices. The primary targets of these projections include the nucleus reticularis pontis oralis and nucleus reticularis gigantocellularis of the medulla, which give rise to the medial (green) and lateral (blue) reticulospinal tracts, respectively, and play important roles in regulating muscle tone.
Autonomic (and Higher-Order Visceral Regulatory) Regions
The reticular formation receives autonomic inputs from different sources. One set of sources includes primary afferent fibers contained in cranial nerves (CN) IX and X. The other source includes fibers arising from higher-order autonomic integrative regions, which include the hypothalamus, as well as parts of the limbic system.
The signals arise from the aortic and carotid bodies (chemoreceptors) and the aortic arch and carotid sinus (baroreceptors) and terminate in the solitary nucleus. Secondary neurons located in the solitary nucleus then project to the nucleus ambiguus and the ventrolateral medullary depressor areas located in the medullary reticular formation. As noted earlier, this information is important for reflex regulation of blood pressure and respiration.
Forebrain Regions (Hypothalamus and Limbic System). Several different pathways that arise from both lateral and medial regions of the hypothalamus project downstream and make synap-tic connections in different parts of the reticular formation, mainly the midbrain and pons. These regions of hypothala-mus project most heavily to the midbrain PAG and neighboring regions of the dorsal and lateral tegmental fields of the reticular formation. Additional groups of fibers from the amygdala supply the PAG, tegmentum, and lower brain-stem region (in and adjacent to the solitary nucleus). Other limbic structures also contribute fibers to the reticular formation. These include inputs from the prefrontal cortex and amygdala that are mainly directed to the midbrain PAG. These descending inputs provide a higher-order control of central autonomic functions of the brainstem reticular formation.
Efferent Projections
Organizational Considerations
As mentioned earlier, the reticular formation receives a variety of inputs from disparate regions of the central nervous system (CNS) that mediate different functions. The varied functions of the reticular formation are even more dramatically reflected by the nature of the outputs. Features concerning the basic organization of the neurons in the reticular formation should be pointed out before describing the output pathways of the reticular formation.
1. The reticular formation contains cells whose axons travel long distances. Some ascend to the forebrain, and others descend to the spinal cord or project to the cerebellum.
2. The efferent fibers that travel long distances arise from the medial two thirds of the reticular formation, and those that travel only short distances as interneurons lie mainly in the lateral third of the reticular formation.
3. The main dendritic branches of the neurons are oriented in a plane perpendicular to the long axis of the brainstem (Fig. 23-6). This arrangement increases the probability that ascending and descending fibers of the reticular formation will make synaptic contact with other regions of the CNS.
4. Cells situated in the medial two thirds of the reticular formation of the medulla and pons give rise to bifurcating axons that travel for long distances in both directions (Fig. 23-7). By virtue of axon collaterals, each of these neurons can make synaptic contact with the other, thus providing an additional (sensorimotor) integrating mechanism by which signals transmitted downstream from the reticular formation can be synchronized with those projecting upstream.
FIGURE 23-6 Ascending and descending axons in the reticular formation. A sagittal section from the brainstem displays large cells in the magnocellular region of the reticular formation. Shown in this illustration is a neuron that bifurcates into an ascending and a descending branch. The branches give off collaterals to the structures adjacent to the reticular formation as well as to other nuclei of the reticular formation.
FIGURE 23-7 Different types of neurons in reticular formation can influence other regions along the neuraxis of the central nervous system. (A)The long descending neuron on the left (shown in red) gives off a collateral that makes synaptic contact with another neuron (shown in blue) that contains a long ascending axon. (B) Alternatively, a single neuron may bifurcate, giving rise to both long ascending and descending branches.
Pathways to Regions Mediating Sensory Functions and Effects on Cortical Excitability Levels
There are at least three ways in which the reticular formation can modulate sensory functions and cortical excitability. The first two mechanisms involve projections from the reticular formation to the intralaminar nuclei of the thalamus, and the third mechanism involves direct projections from monoaminergic neurons of the reticular formation to the cerebral cortex.
Consider first the projections from the reticular formation to the thalamus. Fibers from nuclei within the reticular formation project to nonspecific thalamic nuclei, including the centromedian and parts of the ventral anterior nucleus (VA), whose properties mimic those of a nonspecific thalamic nucleus.In turn, these nonspecific thalamic nuclei project to wide regions of cortex directly or indirectly through a synapse in the VA (Fig. 23-8). Although the projection from the VA to the cortex is extensive, its projection is directed principally to the frontal lobe. Because of the widespread projections, activation of the nonspecific tha-lamic nuclei by the reticular formation can lead to changes in cortical neuron excitability levels.
There is a second, alternative mechanism that could also be operative. Neurons in nonspecific thalamic nuclei are known to make synaptic contact with specific thalamic nuclei (Fig. 23-9). Here, nonspecific thalamic nuclei can modify sensory transmission at the level of the thalamus by interacting with specific thalamic nuclei before the signals can reach sensory regions of the cerebral cortex.
Evidence for a functional relationship between the reticular formation and cerebral cortex comes from electrical stimulation studies in which low-frequency stimulation of nonspecific thalamic nuclei produces a distinctive cortical electrical pattern called a recruiting response. This response is characterized by a surface negative wave, which reaches a maximum amplitude rapidly and then slowly decreases in size. Additional stimulation results in a waxing and waning of the cortical wave. The behavioral response noted from such stimulation of the nonspecific thalamus is that the patient has a drowsy appearance. In contrast, electrical stimulation of the brainstem reticular formation suppresses the recruiting response as well as the electroencephalographic (EEG) response that occurs during quiet, sometimes drowsy states referred to as the alpha rhythm (Fig. 23-10).1 These findings provide evidence for the view that modulation of cortical excitability levels involves inputs to the nonspecific thalamus from the retic-ular formation that are ultimately relayed to the cortex (through the nonspecific thalamus).
Thus, one role of the reticular formation is to provide activation of the cerebral cortex (Fig. 23-11). The process of arousal is highly important because it serves to change excitability levels (i.e., prime the sensory [and other] neurons) of the cortex so that they will become more receptive to other sensory inputs that reach the cerebral cortex through the classical ascending sensory pathways. For example, upon awaking to the sound of fire engines, there is activation of the reticular formation by descending cortical fibers and additional auditory signals (i.e., the continued sounding of the fire engines). This produces cortical desynchronization (beta rhythm) and enables the individual to respond in an appropriate way that generates conscious awareness of these stimuli.
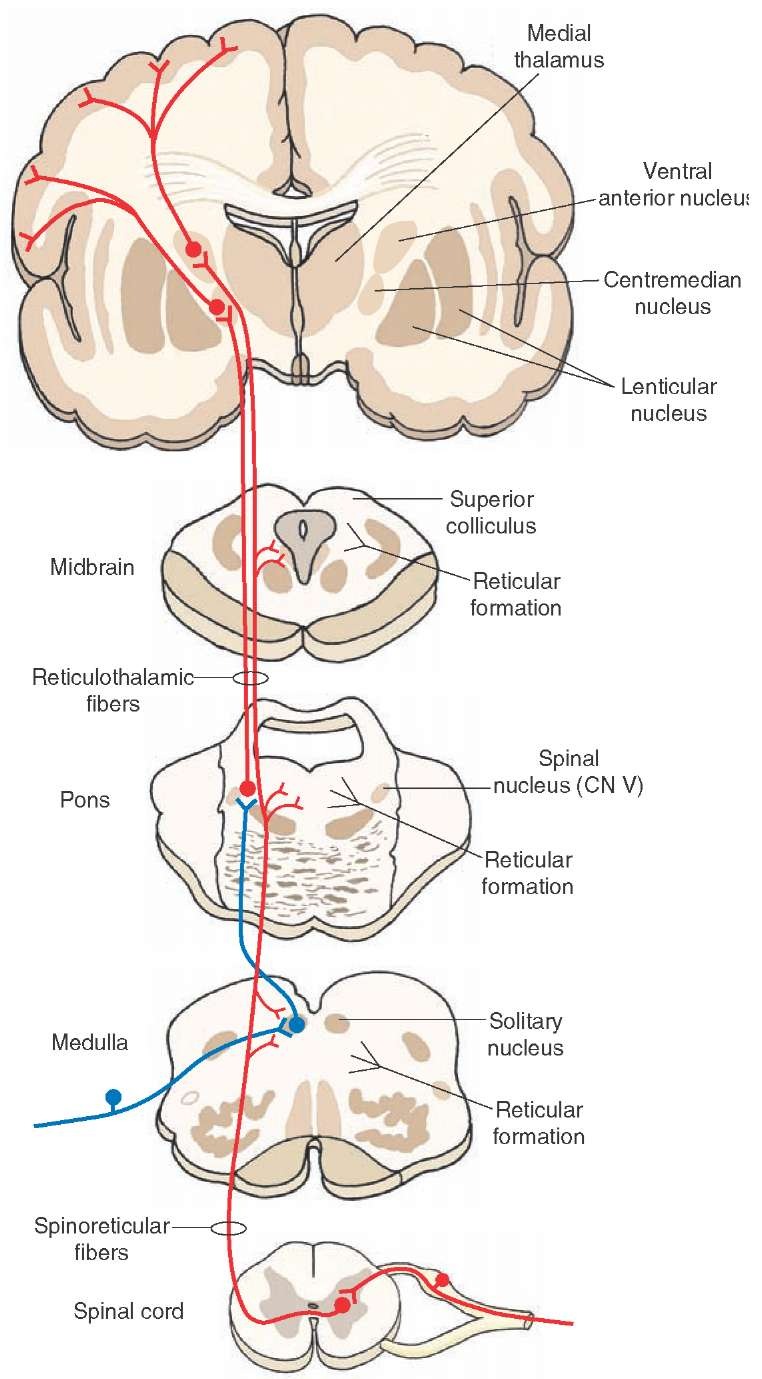
FIGURE 23-8 The ascending connections of the reticular formation and inputs into the reticular formation from lower levels of the central nervous system. The reticular formation receives spinoreticular fibers (shown in red). The ascending reticular fibers project either directly to the intralaminar nuclei (shown in red) or indirectly through an interneuron from the solitary nucleus to the dorsolateral pons first (shown in blue); neurons from intralaminar nuclei then project directly to the cortex (shown in red) or to specific thalamic nuclei, which then project to the cerebral cortex (not shown in this diagram). By either direct or indirect routes, inputs from the reticular formation can influence cortical activity and the transmission of sensory signals to the cortex. CN = cranial nerve.
FIGURE 23-9 Cross section through the thalamus. Note the short efferent projections of a nonspecific thalamic nucleus, the centromedian nucleus (CM), to specific thalamic nuclei, such as the ventral posterior lateral (VPL), ventral posteromedial (VPM), and ventrolateral (VL) nuclei. IC = internal capsule.
It should further be pointed out that the reticular formation can also provide inhibitory modulation of sensory signals.This pathway originates in the midbrain PAG; it is enkephalinergic, and an enkephalinergic neuron synapses on serotonin neurons in the raphe magnus. This nucleus gives rise to a descending serotonin neuron that reaches the dorsal horn of the spinal cord and synapses on another enkephalin neuron, which ultimately modulates the primary afferent nociceptive pathway at this level of the spinal cord. It is possible that the reticular formation further modulates other sensory inputs, thus enabling us to "filter out" unwanted sensory information so that we can more clearly focus on more critical stimuli.
FIGURE 23-10 Tracings of a normal electroencephalogram. The locations of the recording electrodes, shown on the left side of the figure, are taken from an individual who is quiet but awake. Initially, the subject displays an alpha rhythm during the quiet period, but when he opens his eyes (shown by the blink artifacts), the alpha rhythm is replaced by a beta rhythm.
FIGURE 23-11 Electrical stimulation of the reticular formation. Electroencephalographic (EEG) recordings from the studies of Moruzzi and Magoun of the activating patterns of the brainstem reticular formation on the cortex. The four tracings are recorded over different parts of the cerebral cortex of the cat. The arrows pointing up and down indicate the onset and offset of electrical stimulation. Note the desynchronization of the EEG during stimulation followed by a return to a synchronized pattern after stimulation is terminated.
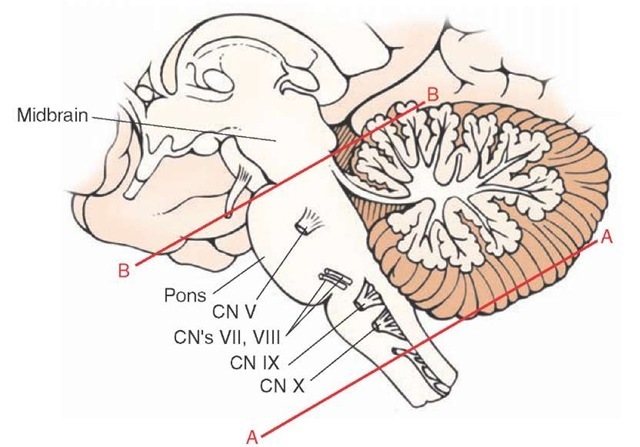
That cortical excitability is dependent on the capacity of the reticular formation to receive sensory information was demonstrated many years ago in experimental studies conducted in the cat. The studies showed that, if one produces a high spinal transection, called an encephale isole preparation (Fig. 23-12), the cortical arousal pattern is retained. If, on the other hand, one produces a transection rostral to the trigeminal nerve (usually a mid-collicular cut), called a cerveau isole preparation (Fig. 23-12), the EEG pattern that results is characteristic of a sleeping animal. After the spinal transection, sensory inputs to the reticular formation from cranial nerves remain largely intact. The only sensory loss is from secondary sensory fibers ascending from the spinal cord. However, following a mid-collicular cut, sensory inputs from both the spinal cord and most sensory cranial nerves are eliminated, especially those inputs from the trigeminal and auditory nerves.
These findings led to a passive theory concerning the basis for states of arousal and sleep. In this view, wakeful-ness results from activation of the reticular formation. Hence, the term "reticular activating system" was coined to describe this mechanism. Sleep was thought to be merely the absence of reticular activation or the shutting off of the reticular formation. But over the last several decades, this notion is no longer accepted. Instead, stages of sleep and wakefulness result from a dynamic interaction between different transmitter systems in the reticular formation as well as the activity of neuronal groups in other regions such as the hypothalamus.
The third way by which the reticular formation can influence sensory transmission is by direct projections from monoamine and cholinergic neurons of the brainstem to the cerebral cortex and other regions of the forebrain.In brief, the key neuro-nal groups include: (1) the raphe neurons, which give rise to serotonergic projections; (2) the nucleus locus ceruleus, which gives rise to noradrenergic projections; (3) cholin-ergic neurons from the region of the pedunculopontine nucleus, which project to the forebrain, and (4) the ventral tegmental area, which contains dopaminergic neurons that project to the entire forebrain with the exception of the neostriatum (which is supplied by the pars compacta of the substantia nigra).
FIGURE 23-12 Experimental transection of the brainstem. Lateral view of the brainstem showing two levels of transection: (line A) near spinal cord-medulla juncture (encephale isole); and (line B) upper pons, rostral to the trigeminal nerve (cerveau isole). An alert cortical electroencephalo-graphic pattern characteristic of a beta rhythm can still be obtained after a cut shown in "A" but not in "B" because, after a transection of the spinal cord-medulla border, sensory information from cranial nerves is preserved, whereas this is not the case after a high pontine transection. CN = cranial nerve.