Learning and Memory Functions of the Hippocampal Formation
There is abundant literature concerning both animal and human studies that indicates that the hippocampal formation plays a significant role in learning processes. In animals, a number of different types of studies have been carried out that link the hippocampus with learning and memory functions. One of the earliest studies demonstrated that a hippocampal theta rhythm (a slow wave of 4-7 Hz) appears as an animal is approaching a goal and during different phases of classical or operant conditioning. Other studies showed that animals with hippocampal lesions persevere by responding in the same way in a learning situation even if the responses are incorrect. If animals are required to delay their responses for a fixed period of time, animals with hippocampal lesions fail to do so and respond during the period when no responses should be made.
The phenomenon of spatial learning (spatial memory) has also been associated with the hippocampal formation. Normally, a rat can learn to enter the correct arm of a Y-maze or the correct series of arms in a more complicated maze such as an eight-armed radial maze. Animals sustaining hippocampal lesions consistently fail to approach the correct arm of the maze and consistently make the same or similar kinds of errors. Other related studies have suggested that a cognitive map exists within the hippocampal formation. The researchers have based their conclusion on the fact that individual hippocampal cells change their discharge patterns as the animal moves to different parts of the cage or when it is placed in different arms of a radial maze.
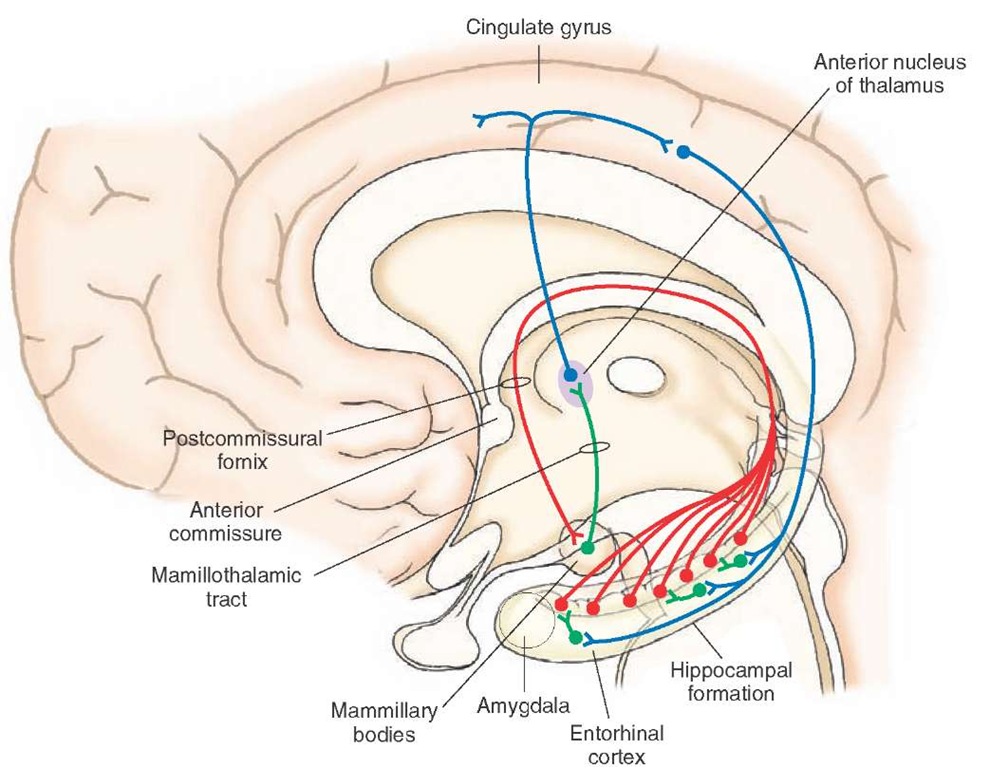
The human literature is equally extensive with respect to learning and memory functions. One of the earliest reported disorders, called Korsakoff’s syndrome, is a memory disorder in which the patient displays amnesia (memory loss) of both anterograde and retrograde memory. Anterograde memory consists of memory loss that occurs after hippocampal damage, whereas retrograde memory loss refers to loss of memory that occurs before damage to the hippocampus. In Korsakoff’s syndrome, the patient experiences a great deal of difficulty in recalling events in the recent past and handling and retaining new information as well as remembering those events that took place in the distant past. Korsakoff’s syndrome is typically associated with the toxic effects of alcohol or from a vitamin B deficiency. Neurons in the hippocampal formation and other parts of the Papez circuit (i.e., the pathway linking the hippocampal formation, especially the mammillary bodies, anterior thalamic nucleus, and cingulate gyrus [Fig. 25-4]) seem to be particularly affected.
Patients sustaining hippocampal lobectomies display perseveration of response tendencies and further display a short-term memory disorder. In such instances, the patient displays anterograde amnesia, but retrograde amnesia is less severe, with little diminution of intellectual abilities. The patient can remember events that took place in the distant past but cannot remember events that occurred recently. For example, if a patient is required to select a geometric form from another series of objects that he was shown 1 minute earlier, he is unable to perform that task. Such a patient may also have difficulty in reading, in that he is unable to remember a previously read line. In contrast, a normal population has little difficulty in successfully completing these tasks.
The following is an example of a memory disorder associated with hippocampal ablation. A patient received a bilateral temporal lobectomy (involving much of the hip-pocampal formation on each side of the brain) to eliminate the spread of severe seizure activity. He was able to remember such facts as the place where he lived years earlier, but he could not remember where he recently moved after the surgery. Similarly, he was able to recall other facts in his distant past, such as people and songs that he had known, but he was unable to remember the people who he met or music that he heard after the surgery. Surprisingly, other forms of intellectual abilities remained intact. He was able to show normal levels of abstract reasoning and perform equally well on related forms of intelligence tests.
These results suggest that one of the functions of the hippocampal formation is to contribute to the consolidation of memory functions by transferring short-term memories to long-term memories. Adequate explanations of the mechanisms underlying the role of the hippocampal formation in memory functions are still lacking in the literature. It is reasonable, however, to suggest that the hippocampal formation normally receives, processes, and categorizes sensory information during learning. Thus, lesions of the hippocampal formation would likely disrupt these events and further interfere with related attentional mechanisms required for the processing and storage of sensory events (in different regions of the cerebral cortex) that are critical for conditioned learning to take place. This kind of loss may be reflected in patients with hippoc-ampal lesions by their inability to encode, categorize, and abstract relevant information necessary for the learning process and, thus, prevents new long-term memories from becoming established.
FIGURE 25-4 Papez circuit. In the Papez circuit, hippocampal fibers project to the mammillary bodies, which, in turn, project through the mammillotha-lamic tract to the anterior nucleus. The anterior thalamic nucleus then projects to the cingulate gyrus, and the axons of the cingulate gyrus then project back to the hippocampal formation.
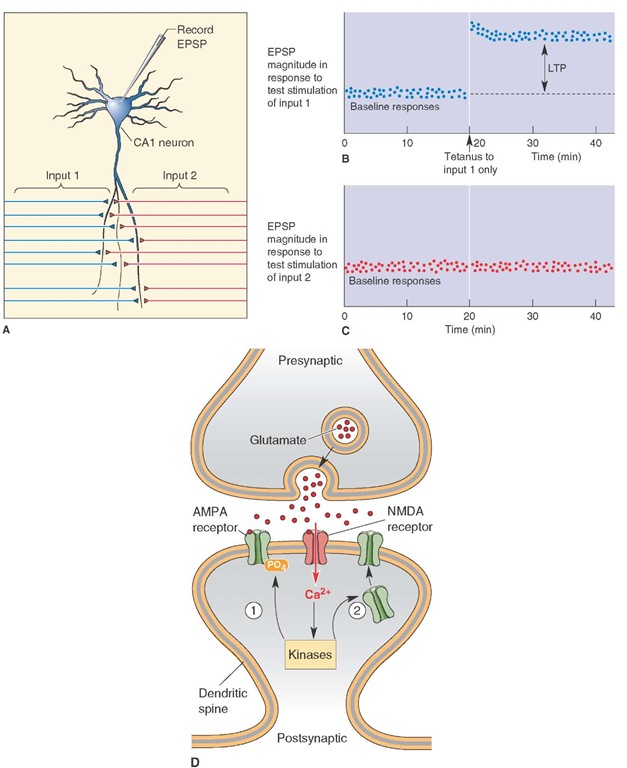
One hippocampal mechanism that has been proposed as a model for memory consolidation is called long-term potentiation (LTP). It represents a change in synaptic strength as a manifestation of synaptic plasticity. LTP can be produced by stimulating fibers that make excitatory connections with hippocampal pyramidal cells. For example, short bursts of high-frequency stimulation of the entorhinal afferents or Schaffer collaterals to hippocampal pyramidal cells over a period of time result in an increased efficiency of the CA1 pyramidal cell response. The increase in excitatory response potency in pyramidal cells that lasts over a period of many minutes and even weeks may constitute a cellular mechanism by which learning takes place (Fig. 25-5, A-C). The suspected synaptic mechanism is believed to involve the release of glutamate that binds to N-methyl-D-aspartate (NMDA) receptors (Fig. 25-5D). This conclusion is based on the fact that NMDA receptor blockade in the hippocampus eliminates LTP. The suggested basis for these events may be as follows. During low-frequency stimulation of the hippocampus, glutamate is released from the Schaffer collaterals and binds to both NMDA and non-NMDA receptors. However, the presence of magnesium blocks the NMDA channels. During high-frequency stimulation, magnesium is released from NMDA channels, and current then flows through both NMDA and non-NMDA channels. Apparently, the essential feature here is that NMDA channels are highly permeable to calcium. Therefore, when these events open calcium channels, there is a likely increase in synaptic efficiency as well as the number of terminals present at that synapse, reflecting a functional change at the synapse during LTP.
Septal Area
Histology
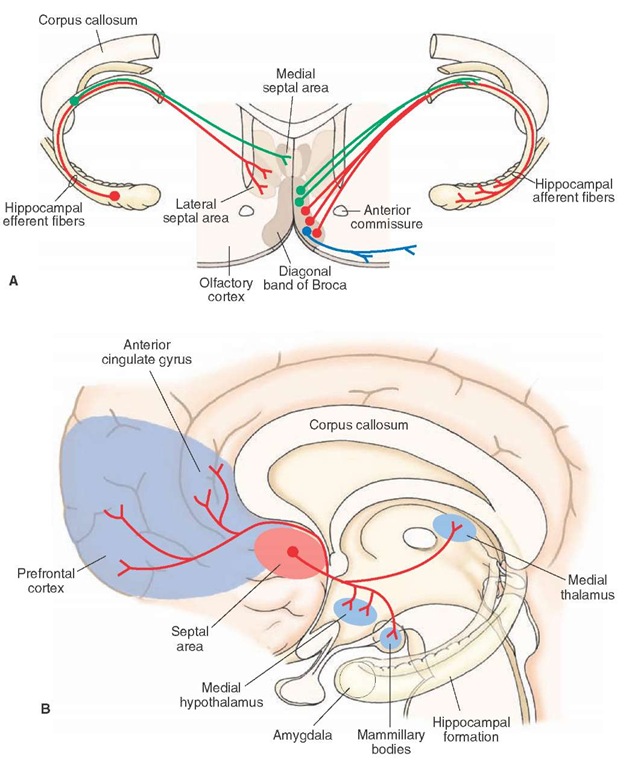
In animals, there exists a dorsal septal area, which includes the lateral septal nucleus and medial septal nucleus. The medial septal nucleus becomes continuous with the vertical limb of the diagonal band of Broca, which lies ventral to the dorsal septal area and is referred to as the ventral septal area (Fig. 25-6A). In humans, a dorsal septal area is not present, and the corresponding area is relegated to the region of the ventral septal area. Two cell groups are sometimes associated with the septal area. These include: (1) the bed nucleus of the stria terminalis, which lies adjacent to the ventrolateral aspect of the lateral septal nucleus and (2) the nucleus accumbens, which lies just lateral to the vertical limb of the diagonal band of Broca (Fig. 25-6A).
FIGURE 25-5 Long-term potentiation (LTP). (A) Activity within the CA1 field of hippocampus is recorded with a microelectrode following alternate stimulation of inputs 1 and 2. (B) In this experiment, high-frequency (tetanus) stimulation applied in input 1 at the time indicated by the arrow potentiated the response as shown in the time period to the right of the arrow. (C) This graph shows the specificity of the response because there was no change in response following stimulation of input 2. EPSP = excitatory postsynaptic potentials. (D) Calcium (Ca2+), which enters through NMDA (N-methyl-D-aspartic acid) receptors and activates protein kinases, can induce LTP by either altering the efficiency of a-amino-3-hydroxyl-5-methyl-4-isoxazole-propionate) AMPA receptors or inducing new AMPA receptors.
FIGURE 25-6 Connections of the septal area. (A) Topographically organized projections from the hippocampal formation to the septal area (left side) and topographically arranged efferent projections from the diagonal band of Broca to the hippocampal formation (rightside) (B) Diagram illustrates other projections from the septal area to the medial hypothalamus, mammillary bodies, medial thalamus, prefrontal cortex, and anterior cingulate gyrus.
Afferent Connections
The septal area receives sensory afferent fibers from the medial olfactory stria, monoaminergic systems of the brain-stem, hippocampal formation, amygdala, and feedback signals from the lateral hypothalamus. The monoaminergic inputs presumably serve to modulate septal neuronal activity, as do the olfactory inputs. As previously pointed out, the septal area serves principally as a relay of the hippoc-ampal formation to the hypothalamus. Thus, the topographically organized inputs from the hippocampal formation (Fig. 25-6A) are considered to be highly significant.
Efferent Connections
The primary efferent projections of the septal area are directed on the hypothalamus and hippocampal formation (Fig. 25-6B). The projections to the hypothalamus arise primarily from the lateral septal nucleus and contribute significant quantities of fibers to the descending component of the medial forebrain bundle. These fibers terminate mainly in the medial hypothalamus. In this manner, the hippocampal formation uses the septal area as a relay nucleus to modulate functions of the hypothalamus.
The hippocampal formation and septal nuclei are reciprocally connected. We have previously pointed out that the hippocampal formation projects topographically to the septal area. The nuclei of the diagonal band, which receive inputs from the lateral septal nucleus, also project topographically back to the hippocampal formation in such a manner that neurons situated most medially project to that part of the hippocampal formation that is located near the septal pole, whereas neurons situated progressively more laterally within the diagonal band project to progressively more ventral and anterior regions that approach the temporal pole of the hippocampal formation (Fig. 25-6B).
The nuclei of the diagonal band of Broca have widespread projections that make synaptic connections with other parts of the limbic system (Fig. 25-6B). They project to olfactory, prefrontal, and anterior cingulate cortices as well as to the amygdala, mammillary bodies, habenular complex, and mediodorsal thalamic nucleus. Accordingly, the nuclei of the diagonal band parallel the projection patterns of the subicular cortex in that both regions are able to communicate with much of the limbic system, thus allowing neurons of the septal-hippocampal axis to communicate and synchronize their activity with much of the limbic system and related nuclei.
Functions of the Septal Area
Because the septal area is reciprocally related to the hip-pocampal formation, it serves not only as a relay for the transmission of hippocampal impulses to the hypothala-mus, but also as a feedback system to the hippocampal formation. Therefore, it is understandable that the functions of the septal area are highly similar to those of the hippoc-ampal formation. Like the hippocampal formation, the septal area has been implicated in the control of functions normally attributable to the hypothalamus, such as aggression, rage, autonomic functions, self-stimulation, and drinking behavior. For example, lesions of the septal area in rats cause them to be highly irritable, aggressive, easily startled, and very difficult to handle. This syndrome is referred to as septal rage. Electrical stimulation of the sep-tal area of the cat modulates aggressive behavior, cardiovascular responses, and single-cell activity in the hypothalamus. Electrical stimulation of the septal area also modulates self-stimulation and drinking behavior elicited from the lateral hypothalamus.
The neuroanatomical basis for these functions of the septal area can be attributed to the septal area’s direct projections to the hypothalamus. Along these lines, direct septal-hypothalamic projections can also account for the modulatory effects that the septal area exerts on the endocrine system via the hypothalamic-pituitary axis. For example, stimulation of the septal area suppresses ACTH secretion and adrenal activity in the rat. Conversely, lesions of the septal area facilitate ACTH release.
The pathway from the septal area to the hippocampal formation may function in ways other than as a feedback mechanism. It has been shown that the nuclei of the diagonal band likely serve as the origin of the hippocam-pal theta rhythm. Moreover, the septal-hippocampal pathway may also contribute inputs that significantly contribute to some of the learning functions normally associated with the hippocampal formation. That such a mechanism may exist is suggested from behavioral studies in which lesions of the septal area impair passive avoidance learning (which also occurs with damage to the hippocampal formation).