Individual Small Molecule Neurotransmitters
In the following sections, important information regarding the synthesis, removal, distribution, and physiological and clinical significance is discussed for selected small molecule neurotransmitters.
Acetylcholine
Synthesis
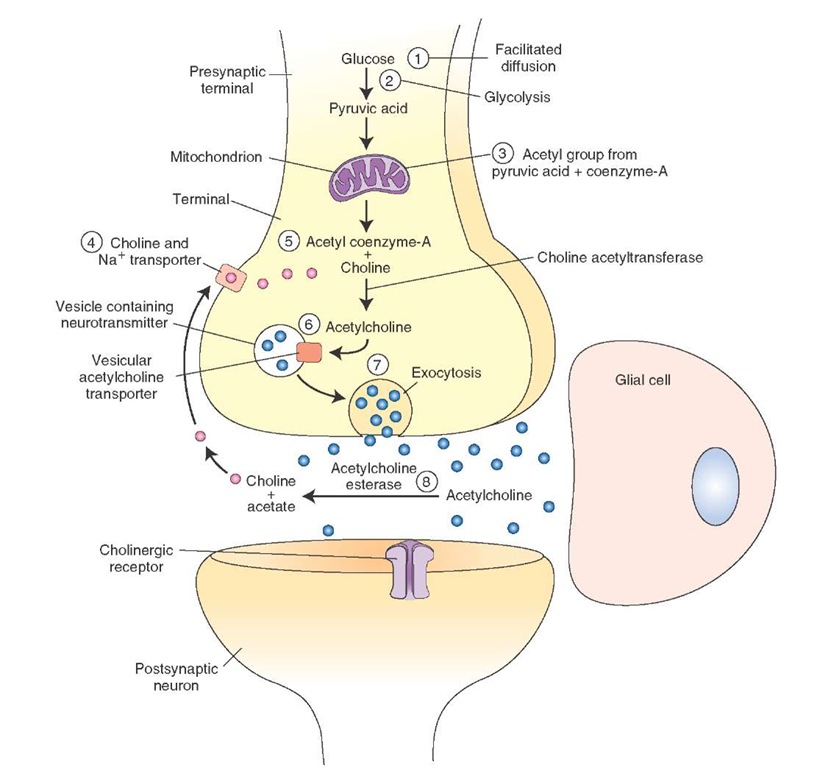
The following steps are involved in the synthesis and release of acetylcholine (Ach [Fig. 8-5]).
1. Glucose enters the nerve terminal by passive transport (facilitated diffusion).
2. Glycolysis occurs in the neuronal cytoplasm, and pyru-vate (pyruvic acid) molecules are generated.
3. Pyruvate is transported into the mitochondria, and an acetyl group derived from pyruvic acid combines with coenzyme-A present in the mitochondria to form ace-tylcoenzyme-A, which is transported back into the cytoplasm.
4. Choline, the precursor for Ach, is actively transported into the neuronal terminal from the synaptic cleft via Na+ (sodium) and choline transporters.
5. Ach is synthesized in the cytoplasm of the nerve terminal from choline and acetylcoenzyme-A in the presence of an enzyme, choline acetyltransferase.
6. Ach is then transported into vesicles and stored there.
7. It is then released into the synaptic cleft by exocytosis and hydrolyzed by acetylcholinesterase (see "Removal" below).
FIGURE 8-5 Steps involved in the synthesis and release of acetylcholine. Na+ = sodium.
Removal
High concentrations of an enzyme, acetylcholinesterase, are present on the outer surfaces of the nerve terminal (prejunctional site) and the effector cell (postjunctional site). Acetylcholinesterase is synthesized in the endo-plasmic reticulum of neuronal cell bodies and major dendrites and is transported to the presynaptic terminal membrane by microtubules. This enzyme hydrolyses Ach in the junctional extracellular space; choline liberated in this reaction re-enters the nerve terminal and is again used for the synthesis of Ach.
Distribution
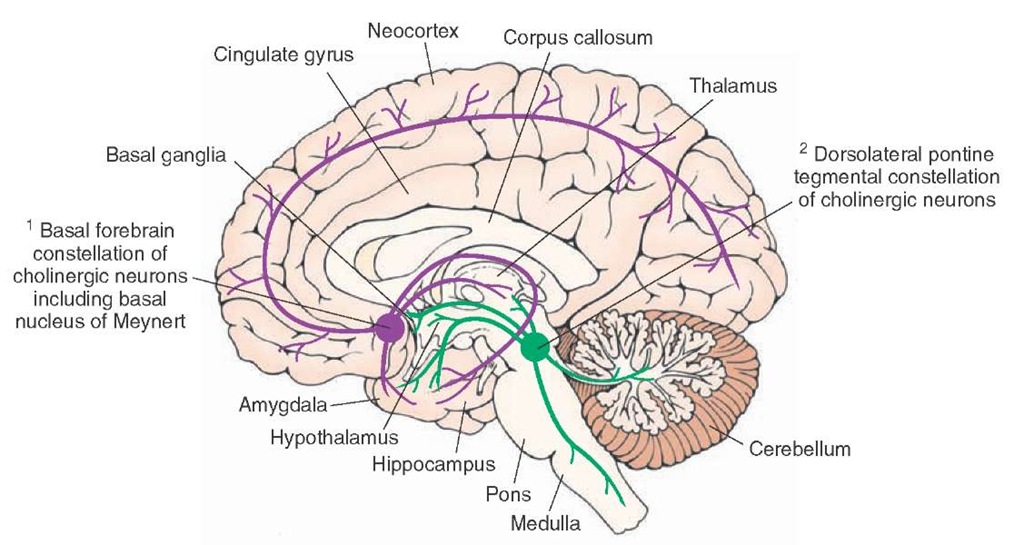
There are two constellations of cholinergic neurons (Fig. 8-6).
1. The basal forebrain constellation is located in the telen-cephalon, medial and ventral to the basal ganglia. It includes the basal nucleus of Meynert, which provides cholinergic innervation to the entire neocortex, amygdala, hippocampus, and thalamus. The medial septal nuclei provide cholinergic innervation to the cerebral cortex, hippocampus, and amygdala.
2. The second constellation includes cholinergic neurons located in the dorsolateral tegmentum of the pons that project to the basal ganglia, thalamus, hypothalamus, medullary reticular formation, and deep cerebellar nuclei.
Physiological and Clinical Considerations
Cholinergic neurons in the dorsolateral tegmentum of pons have been implicated in the regulation of forebrain activity during cycles of sleep and wakefulness. Cholinergic neurons of the basal forebrain constellation are involved in learning and memory and have been implicated in Alzheimer’s disease. In this disease, there is extensive neural atrophy, especially in the cortex and hippocampal formation. Patients with this disease suffer from memory loss, personality change, and dementia. There is a dramatic loss of cholinergic neurons in the basal nucleus of Meynert and Ach in the cortex of these patients. These observations prompted attempts to treat Alzheimer’s disease by Ach replacement therapy. For example, treatment with donepezil (Aricept), an ace-tylcholinesterase inhibitor, is indicated for mild to moderate dementia in patients with Alzheimer’s disease. However, these attempts have not shown dramatic results in relieving the symptoms of the disease, indicating that the mechanisms of neuronal degeneration in Alzheimer’s disease must involve multiple transmitter systems.
FIGURE 8-6 Major cholinergic cell groups. Note two major constellations of cholinergic neurons: cholinergic neurons located in the basal forebrain constellation, including the basal nucleus of Meynert, and cholinergic neurons located in the dorsolateral tegmentum of the pons.
Excitatory Amino Acids: Glutamate
Glutamate is discussed as an example of the excitatory class of neurotransmitters. It occurs in high concentrations in the brain. Although it plays a role in other functions, such as synthesis of proteins and peptides, glutamate is considered to be an important excitatory amino acid because it fulfills the criteria for a substance to be accepted as a neurotransmitter.
Synthesis
Glutamate is synthesized in the brain by two processes. In one process, glucose enters the neuron by facilitated diffusion and is metabolized via the Krebs (tricarboxylic acid) cycle. a-oxoglutarate generated during the Krebs cycle is transaminated by a-oxoglutarate transaminase to form glutamate.
The following steps are involved in the second process of the synthesis of glutamate (Fig. 8-7).
1. and 2. Nerve terminals and glial cells reuptake the glutamate released from the nerve terminals via gluta-mate transporters located in their cell membranes.
3. In the glia, glutamate is converted into glutamine by an enzyme, glutamine synthetase.
4. Glutamine is transported out of the glia into the neuro-nal terminal via glutamine transporters located in the glial and neuronal terminal membranes.
5. In the neuronal terminal, glutamine is converted into glutamate by an enzyme, glutaminase. Glutamate is taken up into the vesicles by active transport, stored, and subsequently released by exocytosis. Released gluta-mate is then actively taken up by the glia and neuronal terminals via glutamate transporters. In the neuronal terminal, it is repackaged into the vesicles for subsequent reuse. In the glia, it is converted into glutamine as described earlier.
Removal
As described earlier, glutamate transporters are located on the plasma membrane of the presynaptic nerve terminal and on glial cells. Glutamate is taken up by a high-affinity, sodium-dependent, re-uptake mechanism into the nerve terminals and glial cells via these transporters.
Physiological and Clinical Considerations
Some of the important physiological and clinical considerations relevant to glutamate are as follows.
1. Glutamate has been implicated as a transmitter in a variety of circuits in the brain. For example, excitatory amino acids may be involved in learning and memory processes, as well as motor functions.
2. Glutamate has also been implicated in some chronic neuropathological conditions such as amyotrophic lateral sclerosis ([ALS] also known as Lou Gehrig’s disease), which is characterized by degeneration of the motor neurons in the anterior horn of the spinal cord, brainstem, and cerebral cortex. Patients suffering from this disease exhibit widespread muscle fasciculations, flaccid weakness, and atrophy of the muscles. The mechanism of the involvement of glutamate in the symptoms of ALS is not clearly known.
3. Prolonged stimulation of neurons by excitatory amino acids results in neuronal death or injury. This effect is known as excitotoxicity, in which a loss of postsynaptic neurons predominates. Neuronal loss due to excitotox-icity has been implicated in cerebrovascular stroke. Occlusion of arteries supplying the brain results in brain ischemia. Concentrations of excitatory amino acids (glutamate and aspartate) increase in the extracellular spaces in the brain, perhaps due to slowing of their uptake by neurons and glia. The neurons are excited excessively, and a chain of events, not fully identified yet, causes neuronal death. Excessive intracellular Ca2+ concentrations have been implicated in the neuronal death due to excitotoxicity.
FIGURE 8-7 Steps involved in the synthesis and release of glutamate.
4. Excitotoxic action of glutamate has been implicated in Alzheimer’s disease. One of the mechanisms of the loss of neurons concerned with memory in this disease is believed to be overexcitation and, finally, loss of these neurons by glutamate-induced activation of N-methyl-D-aspartic acid (NMDA) receptors (described later in this topic in the "Ionotropic Receptors" section). Memantine (Namenda), an NMDA receptor antagonist, is reported to mitigate this effect and slow down the symptoms of the disease.
Inhibitory Amino Acids
In this section, gamma aminobutyric acid and glycine, two main inhibitory amino acid neurotransmitters, will be discussed.