Composition of Peripheral Nerves
Each peripheral nerve consists of the following components:
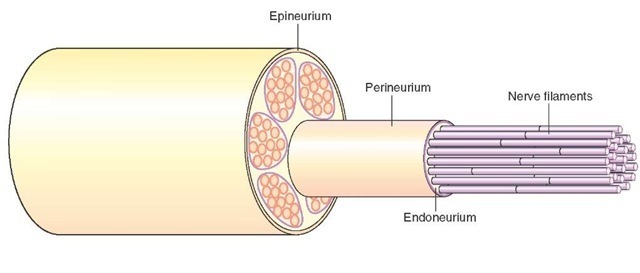
1. Epineurium, which consists of a dense connective tissue layer enclosing several bundles of nerve fibers (Fig. 5-8);
2. Perineurium, which is a sheath of connective tissue enclosing each bundle of nerve fibers (Fig. 5-8); and
3. Endoneurium, which consists of a loose, delicate connective tissue layer in which nerve fibers are enclosed (Fig. 5-8).
Nerve fibers have been classified into three major groups based on the diameters and conduction velocities (Table 5-1). Peripheral nerves include the following groups of nerves: cranial nerves, spinal nerves, and preganglionic and postganglionic nerves of the autonomic nervous system.The axons in a peripheral nerve may be myelinated or unmyelinated. It should be noted that nerve bundles traversing the CNS (e.g., internal capsule) lack the connective tissue coverings (e.g., epineurium and perineurium) that enclose peripheral nerves.
Clinical Considerations
Disorders Associated With Defective Myelination
Normal conduction of the nerve impulses is dependent on appropriate insulation provided by the myelin sheath surrounding the axons in the CNS and PNS. Demyelination of axons occurs in diseases such as multiple sclerosis and Guillain-Barre syndrome.
Multiple Sclerosis
Guillain-Barre Syndrome
Guillain-Barre syndrome (GBS) is an inflammatory disease in which demyelination occurs in the axons within the peripheral nerves. It should be noted that, in multiple sclerosis, demyelination occurs in the axons within the CNS.
FIGURE 5-7 Myelination in the central nervous system (CNS). Within the CNS, oligodendrocytes form the myelin sheaths around neurons. Several glial processes arise from one oligodendrocyte and wrap around a portion of the axon. The intervals between adjacent oligodendrocytes are devoid of myelin sheaths and are called the nodes of Ranvier.
FIGURE 5-8 Composition of a peripheral nerve: The epineurium, perineurium, endoneurium, and nerve fibers in a peripheral nerve.
TABLE 5-1 General Classification of Nerve Fibers*
|
Type |
Size (mm) |
Conduction Velocity (m/sec) |
Functions |
|
Myelinated |
|||
|
A |
1-20 |
5-120 |
Larger, faster conducting fibers (60-120 m/sec) transmit motor impulses to the skeletal muscle; smaller |
|
A fibers conduct afferent impulses from muscle spindles, Golgi tendon organs, and mechanoreceptors |
|||
|
B |
1-3 |
3-15 |
Afferent and efferent innervation of the viscera |
|
Unmyelinated |
|||
|
C |
<2 |
0.6-2 |
Efferent: postganglionic fibers of the autonomic nervous system |
|
Afferent: impulses of poorly localized pain in the viscera or periphery |
* Other nomenclature for the classification of nerve fibers.
Although the precise cause of GBS is rarely found, the disease is believed to be an immunological reaction resulting from a prior infection or inflammatory process. It is characterized by progressive motor weakness. Clinically, motor and sensory loss potentially affecting the face, limbs, trunk, and diaphragm is manifested. Areflexia may be present because of damage to both reflex arcs of the deep tendon reflex. Inflammatory cells (lymphocytes and mac-rophages) are often found within the peripheral nerves. Additionally, segmental demyelination and Wallerian degeneration (see next section) may be found.Most patients recover. Treatment is aimed at the immunological aspect, with gamma globulin administration and plasma exchange being the most commonly used. Gamma globulin blocks receptors where antibodies attach to cause damage. In plasma exchange, blood is filtered and plasma removed and replaced by albumin so that antibodies causing demy-elination are eliminated.
Neuronal Injury
Injury of the Neuronal Cell Body
The neuronal cell body may be damaged by disease, ischemia (lack of blood supply), or trauma. In the CNS (the brain and spinal cord), the debris produced by neuronal damage is phagocytosed (see below) by microglia. The adjacent fibrous astrocytes proliferate, and the neurons are replaced by scar tissue. In the PNS, macrophages are responsible for the removal of the debris produced by neu-ronal damage, and the scar tissue is produced by the proliferation of the fibroblasts.
Necrotic cell death is caused by acute traumatic injury that involves rapid lysis of cell membranes. Necrotic cell death is different from apoptosis. Apoptosis is defined as a genetically determined process of cell death and is characterized by shrinkage of the cell, cellular fragmentation, and condensation of the chromatin. During the process of formation of tissues from undifferentiated germinal cells in the embryo (histogenesis), more neurons (about 2 times more) are formed than the neurons present in the mature brain. The excess number of neurons is destroyed during the development by apoptosis. The mechanism of apoptosis involves activation of a latent biochemical pathway that is present in neurons and other cells of the body. The cellular debris after neuronal cell death is removed by phagocytosis, which involves transport of solid material into the cells (e.g., microglia) that remove the debris by indentation of the cell membrane of the phagocyte and formation of a vesicle. Pinocytosis is similar to phagocytosis, except that liquid material is removed. Exocytosis involves fusion of a vesicle inside the nerve terminal (e.g., a vesicle containing a neurotransmitter) with the plasma membrane and transportation of the contents of the vesicle outside the nerve terminal.
Axonal Damage
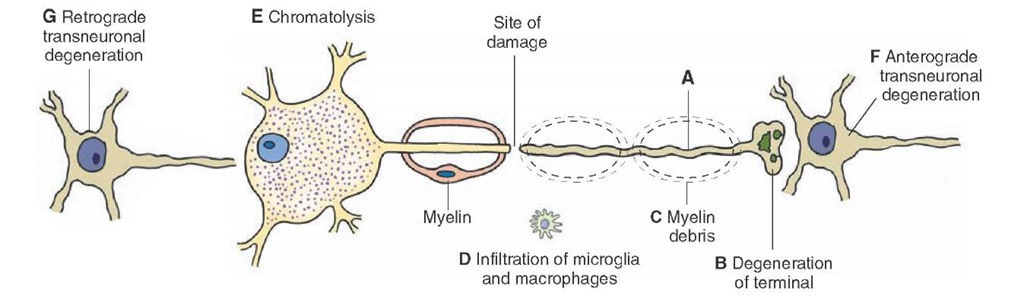
Wallerian Degeneration. Wallerian degeneration refers to the changes that occur distally to the site of damage on an axon. Because protein synthesis occurs primarily in the neuronal cell body, the segment distal to the damaged site on the axon is affected profoundly. Initially, the axon swells up and becomes irregular. Later, the axon and the terminal are broken down into fragments that are phago-cytosed by adjacent macrophages and Schwann cells (Fig. 5-9, A-D). Myelin is converted into fine drops of lipid material in the Schwann cells and is extruded from these cells; it is removed by macrophages in the PNS and microglial cells and invading macrophages in the CNS.
Alterations (similar to those mentioned earlier) may also be present in the proximal segment of the axon up to the first node of Ranvier.
Chromatolysis. Sectioning of an axon may produce changes in the cell body, and if the injury is close to the cell body, the neuron may degenerate. The cell body swells up due to edema and becomes round in appearance, and the Nissl substance gets distributed throughout the cytoplasm. This process is known as chromatolysis (Fig. 5-9, E). The nucleus moves from its central position to the periphery due to edema. The degenerative changes start within hours and are complete within a relatively short time (about a week). Anterograde Transneuronal Degeneration. Anterograde transneu-ronal degeneration occurs in the CNS when damage to a neuron results in the degeneration of another postsynaptic neuron closely associated with the same function (Fig. 5-9, F). For example, damage to an optic nerve results in the degeneration of the lateral geniculate neurons receiving inputs from this nerve. Retrograde Transneuronal Degeneration. Retrograde transneu’ ronal degeneration occurs in neurons sending inputs to an injured neuron. In this situation, terminals of the neuron synapsing with a chromatolytic neuron withdraw and are replaced by processes of glial cells. The neuron, from which the inputs to the chromatolytic neuron arise, eventually degenerates (Fig. 5-9, G).
FIGURE 5-9 Effects of axonal injury. (A-D) Wallerian degeneration. (E) If the injury is close to the cell body, chromatolysis occurs in the cell body. (F) In the central nervous system, the neuron receiving input from a degenerated axon undergoes anterograde transneuronal degeneration, and (G) the neuron, from which the inputs to the chromatolytic neuron arise, degenerates.
Recovery of Neuronal Injury (Regeneration)
In the adult CNS, if the damage is not severe and some neuronal cell bodies are spared, sprouting of axons does occur, but this process ceases within a short time (about 2 weeks). Astrocytes proliferate at the site of injury in a random fashion and form a scar which acts as a barrier for axonal sprouts. Furthermore, astrocytes may not release growth factors that are needed for axonal growth, and oligodendrocytes may release substances that retard axonal growth. In this situation, regeneration of axonal tracts does not occur, and normal functions of the neurons are not restored. However, in peripheral nerves, an axon can regenerate satisfactorily if the endoneurial sheaths are intact. In this situation, the regenerating axons reach the correct destination, and the chances of recovery of function are reasonable. The growth rate of an axon has been estimated to be 2 to 4 mm per day.
Clinical Case
History
Roseanne is a 40-year-old woman who was brought to a local emergency room (ER) due to 7 days of progressive numbness and weakness, first appearing in her toes, then moving proximally to her feet, knees, and hands. She initially ignored the symptoms because she thought that the tingling in her feet was merely her feet "falling asleep." However, the symptoms progressed proximally, and she finally realized that something was wrong when she was unable to climb the stairs of her house and developed increasing shortness of breath.
Examination
Roseanne was short of breath continuously when she arrived at the ER. Her vital capacity (the greatest volume of air that can be exhaled from the lungs after maximal inspiration) was extremely low, which was consistent with severe weakness of the diaphragm and poor oxygenation. Her arms and legs were weak bilaterally, and she had no sensation in her legs or her arms below her elbows. She was able to feel a pin on her upper chest. No deep tendon reflexes could be elicited. Roseanne was transferred to the intensive care unit.
Explanation
Roseanne has a condition called Guillain-Barre syndrome (GBS). GBS is an inflammatory demyelinating disease of peripheral nerves, which may be rapidly progressive. Conduction of action potentials in the affected nerves is either slowed down or blocked due to demyelination. Histologically, inflammatory cells are found within the nerves. Additionally, segmental demyelination and Wallerian degeneration progressing in a proximal direction may be found. Clinically, progressive motor and sensory loss is manifested, potentially affecting the limbs, face, trunk, and diaphragm. This damage also eliminates both reflex arcs of the deep tendon reflexes. GBS is thought to be an immunologic reaction resulting directly or indirectly from a prior infection or inflammatory process, although the precise cause is rarely found. Sometimes, the patient gives a history of a viral infection 2 or 3 weeks prior to the onset of the GBS symptoms. Most patients recover; however,and treatment is aimed at the immunological aspect, with gamma globulin administration and plasma exchange being the most common treatments.
SUMMARY TABLE
Clinical Disorders Associated With Defective Myelination
|
Disorder |
Defect |
Symptoms |
Treatment/Management |
|
Guillain-Barr£ syndrome |
Demyelination of axons in peripheral nerves; immunological reaction resulting from a prior infection or inflammatory process; Wallerian degeneration |
Progressive motor weakness; motor and sensory loss affecting the face, limbs,trunk,and diaphragm;areflexia due to damage of both reflex arcs of the deep tendon reflex; inflammatory cells (lymphocytes and macrophages) found within peripheral nerves |
Gamma globulin (blocks receptors where antibodies attach to cause damage); plasma exchange to reduce or eliminate the antibodies causing demyelination; most patients recover |
|
Multiple sclerosis" |
Demyelination of axons in central nervous system (CNS); autoimmune disease with inflammatory features affecting CNS |
Antibodies attack myelin, which swells and detaches; a scar (sclerosis) develops on nerve fibers, which delays or blocks nerve impulses; finally, nerve fibers degenerate; somatosensory pathways are compromised causing numbness in one or more limbs and tingling or pain in parts of the body; visual pathways are affected (partial or complete loss of vision in one eye at a time and double or blurred vision); disturbances in speech |
Interferon beta-la and interferon beta-lb; glatiramer (Copaxone); natalizumab (Tysabril); mitoxantrone (Novantrone); plasma exchange (plasmapheresis) |
|
Charcot- Marie-Tooth syndrome" |
Demyelination of peripheral nerves; caused by a mutation in one of the con-nexin genes expressed in Schwann cells;failure of formation of gap-junctions, which are necessary for flow of ions and small metabolites between myelin layers |
Demyelination affects both motor and sensory nerves; weakness of the foot and lower leg muscles is typical; patients have a high-stepped gait with frequent tripping or falls; deformities in the foot and digits |
No cure; patients are advised to undergo physical and occupational therapy; nonfatal and patients have normal life expectancy |
Types of Neuronal Injury
|
Injury of the Neuronal Cell Body |
Characteristics |
|
Necrotic cell death |
Caused by acute traumatic injury that involves rapid lysis of cell membranes |
|
Apoptosis |
Genetically determined process of cell death characterized by shrinkage of the cell, cellular fragmentation, and condensation of the chromatin; involves activation of a latent biochemical pathway that is present in neurons and other cells of body |
|
Chromatolysis |
If axonal injury is close to cell body neuron may degenerate, cell body swells up due to edema and becomes round in appearance; Nissl substance gets distributed throughout cytoplasm; nucleus moves from its central position to periphery due to edema |
|
Anterograde transneuronal degeneration |
Occurs in the CNS when damage to a neuron results in the degeneration of another postsynaptic neuron closely associated with the same function |
|
Retrograde transneuronal degeneration |
Occurs in neurons sending inputs to an injured neuron; terminals of neuron synaps-ing with a chromatolytic neuron withdraw and are replaced by processes of glial cells; neuron,from which the inputs to the chromatolytic neuron arise, eventually degenerates |
|
Axonal Injury |
Characteristics |
|
Wallerian degeneration |
Changes that occur distally to the site of damage on an axon; axonal segment distal to the damaged site swells up and becomes irregular; eventually axon and terminal are broken down into fragments that are phagocytosed by adjacent macrophages and Schwann cells; myelin is converted into fine drops of lipid material in the Schwann cells and is extruded from these cells and removed by macrophages in peripheral nervous system and by microglial cells in CNS |
CNS = central nervous system.