Introduction
The perioperative management of paediatric patients undergoing neurosurgical procedures should be based on the developmental stage of the patient. Age-dependent differences in cranial bone development, cerebral vascular physiology and neurological lesions distinguish neonates, infants and children from their adult counterparts. In particular, the central nervous system (CNS) undergoes a tremendous amount of structural and physiological change during the first 2 years of life. Te goal of this topic is to highlight these age-dependent differences and their effect on the perioperative management of the paediatric neurosur-gical patient.
The neonate
Neurological pathologies that require surgery during the neonatal period are mainly due to life-threatening congenital anomalies or intracranial haemorrhage. Tere are a variety of reasons why neonates undergoing neurosurgical procedures present special challenges to anaesthesiologists. For one, neonatal physiology is different from adult physiology. For example, the anaesthesiologist must be mindful of the additional perioperative concerns that newborn cardiac physiology presents during perioperative care. Te possibility ofintracardiac shunts, elevated pulmonary pressures and a stiff ventricle result in fundamentally different perioperative management strategies. In addition to physiological differences, neonates may also have different drug metabolism from adults, especially with hepatically metabolized drugs. Closed claim analysis studies have revealed that neonates are at higher risk for morbidity and mortality than any other age group. Respiratory- and cardiac-related events account for the majority of these complications. Given the urgent nature of many neonatal neurosurgical procedures, a thorough pre-operative evaluation may be difficult, and other coexisting congenital anomalies may not be detected. Tus, thorough understanding of the fundamental differences inherent in neonates is essential.
Anatomy
The diminutive size of the premature and term neonate has a significant impact on the perioperative course. As noted above, neonates and infants are at higher risk for morbidity and mortality than any other age group, with respiratory- and cardiac-related events accounting for the majority of these complications. Anatomic differences between the paediatric and adult airway are primarily due to the size and orientation of the components of the upper airway, larynx and trachea. In addition, neonates and infants have the greatest differences from adults in this respect. However, the configuration of the larynx becomes similar to the adult after the second year of life. Te infant’s larynx is also funnel-shaped and narrowest at the level of the cricoid, making this the smallest cross-sectional area in the infant airway. Tis feature places the infant at particular risk for life-threatening subglottic obstruction secondary to mucosal swelling after prolonged intubation with a tight-fitting endotracheal tube. Te use of a cuffed endotracheal tube should be undertaken with great caution in this age group, and cuff pressures should be monitored carefully, especially when using nitrous oxide (NtO). Te distance between the vocal cords and the carina can be quite short in small babies, and thus the difference between extubation and mainstem intubation can be only a few centimetres. If the infant’s head is flexed for a suboccipital approach to the posterior fossa or the cervical spine, an endotracheal tube can migrate into a mainstem bronchus. Given these conditions, the anaesthesiologist should auscultate both lung fields after the patient is positioned for the surgical procedure to rule out inadvertent intubation of a mainstem bronchus.
Fig. 14.1. Functional anatomy of cranial sutures and fontanelles in neonates and infants. Initially the compliant skull of the neonate will minimize insidious increases in intracranial volume. However, acute increases in intracranial volume (haemorrhage and obstructed ventriculoperitoneal shunt) will lead to rapid rises in intracranial pressure.
The neonatal cranial vault is in a state of flux. Open fontanels and cranial sutures lead to a compliant intra-cranial space (Fig. 14.1). Unlike an adult cranium, the neonatal cranium has a ‘pop-off’ valve when the fontanels are open. Frequent measurement of head circumference is often utilized to assess concerns for expanding intracranial lesions. T e mass ef ect of an insidious haem orrhage is often masked by a compensatory increase in intracranial volume due to widening of the fontanels and cranial sutures. However, like adults, acute increases in cranial volume due to massive haemorrhage or an obstructed ventricular system cannot be attenuated by expansion of the immature cranial vault, which frequently results in life-threatening intracra-nial hypertension .
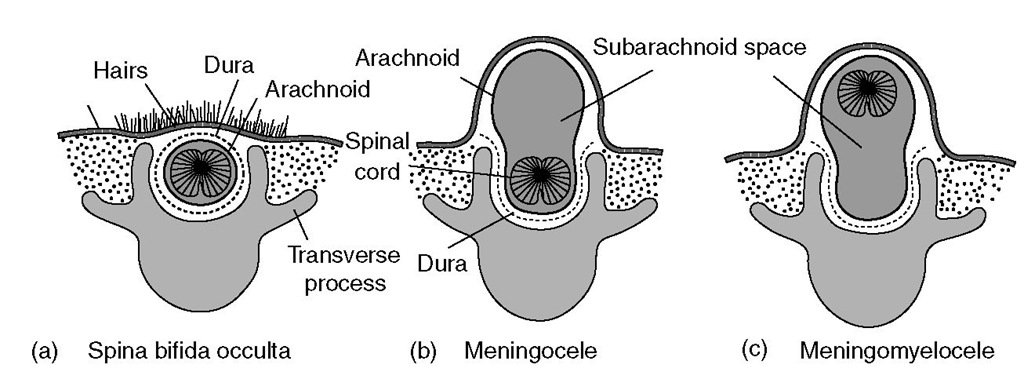
Congenital anomalies of the CNS generally occur as midline defects. Tis dysraphism may occur anywhere along the neural axis (from cranium to sacrum), involving the head (encephalocele) or spine (meningomyelocele) . Te extent of such defects is incredibly varied, and can range from relatively minor defects that affect only superficial bony and membranous structures to more serious defects that include a large segment of malformed neural tissue (Fig. 14.2).
Physiology
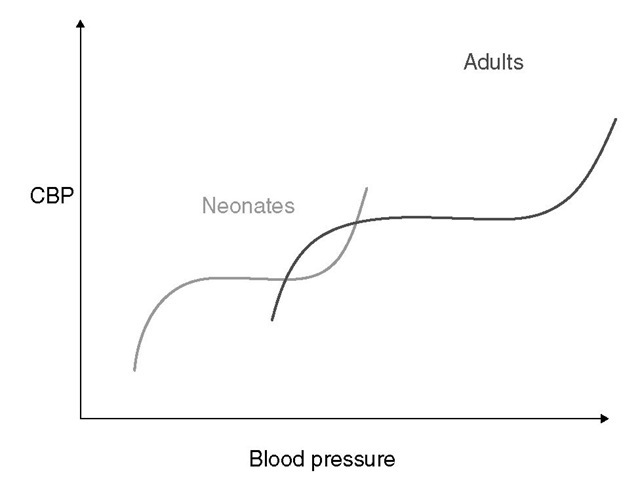
Developmental cerebral blood flow (CBF) is coupled tightly to metabolic demand, and both increase proportionately immediately after birth. Cerebral blood flow peaks between 2 and 4 years and stabilizes at 7-8 years. Tese alterations in CBF mirror changes in neuroanatomical development. Even in the neonate, autoregulatory mechanisms are present. Te autoregu-latory range of blood pressure in a normal newborn is between 20 and 60 mmHg, which reflects the relatively low cerebral metabolic requirements and blood pressure during the perinatal period. More importantly, the slope of the autoregulatory curve is quite steep at the upper and lower limits of the curve, while the flatter portion occurs over a relatively narrow range (Fig. 14.3). Neonates are especially vulnerable to cerebral ischaemia and intraventricular haemorrhage due to this narrow autoregulatory range.
Certain neonates are at an even higher risk of morbidity and/or mortality. Tsuji and colleagues demonstrated that sick premature neonates have a linear correlation between CBF and systemic blood pressure, placing these patients at particular risk for cerebral ischaemia as well as intraventricular haemorrhage due to this direct relationship and a lack of autoregulatory protection. Also, CBF pressure passivity occurs in premature neonates with low gestational age, birthweight and systemic hypotension. Furthermore, Boylan and colleagues reported that high-risk term as well as premature neonates did not demonstrate autoregulation of CBF.
Extreme vigilance and care must be taken when anaesthetizing neonates but particularly with sick pre-m ature infants. Te balance between adequate cerebral perfusion and maintenance of adequate anaesthetic depth can be difficult. It is crucial to take into account the physiological dif erences in this patient population, and thus tight blood pressure control is essential in the management of neonates to minimize both cerebral ischaemia and intraventricular haemorrhage.
Premature and term neonates have functionally immature organ systems. Te immaturity of the renal system is of particular concern during many neuro-surgical procedures where significant fluid shifts may occur. Te neonatal renal system is characterized by a decreased glomerular filtration rate and ability to concentrate urine in response to dehydration states. Tese differences result in diminished excretion of sodium and water, thus limiting the neonate’s ability to compensate for fluctuations in fluid and solute loads. Drugs that are renally excreted may have a prolonged half-life. Furthermore, hepatic function is also diminished in neonates, and metabolism of drugs may be delayed due to decreased activity of hepatic enzymes. Neonates demonstrate a decreased ability to glucuro-nidate drugs, and thus drugs like morphine may have quite extended half-lives in the neonate. Total body water decreases from 85% in premature infants to 65% in adults, while body fat content increases from < 1% in premature infants to 15% in term infants and 35% in adults. Total body protein also follows a similar trend, and therefore hydrophilic drugs have more binding sites and hydrophobic drugs have fewer in infancy. Te constellation of these factors should prompt the clinician generally to decrease the weight-adjusted dose and frequency of administration of drugs given to the newborn. Judicious titration of potent drugs is critical in these patients.
Fig. 14.2. Spinal dysraphism. (a) Spina bifida occulta – skin or skin with hair covers a bony defect only. (b) Meningocele – protrusion of a fluid-filled sac only (no neural tissue present). (c) Meningomyelocele – protrusion of a fluid-filled sac plus neural tissue.
Fig. 14.3. Autoregulation of cerebral blood flow in neonates and adults. The slope of the autoregulatory curve drops and rises significantly at the lower and upper limits of the curve and is shifted to the left in neonates and infants (left curve) when compared with adults (right curve).
Most neonatal surgery is performed on an emergent basis, which increases risk in the perioperative period due to undiagnosed congenital anomalies. Premature neonates may have persistence of the transitional circulation, which is the period of transition from fetal circulation to adult circulation. Of particular concern during this period are the presence of physiological intracardiac shunts (patent foramen ovale and patent ductus arteriosus), relatively elevated pulmonary vascular resistance and a stiff ventricle. Te neonatal heart is characterized by fewer contractile elements and fewer elastic elements. Tis means that the neonatal heart cannot increase cardiac output with volume via the Frank-Starling method and that there is decreased ventricular compliance. Congestive heart failure can occur in neonates with large cerebral arteriovenous malformations and this condition requires aggressive haemodynamic support. More commonly, intracardiac right-to-left shunting occurs through patent ductus arterious or foramen ovalae that have not yet closed.
Finally, the respiratory system in neonates also presents a number of challenges. As with both neurological and cardiac physiology, respiratory physiology in neonates is different from adult patients. Neonates have a lower functional residual capacity, as well as a higher rate of oxygen consumption. Tis can make airway management and particularly rapid sequence inductions challenging. Premature infants may have some degree of lung disease, which can predispose these patients to bronchospasm and significant oxygen desaturations. Management of the neonatal respiratory system may be difficult for a variety of reasons, including the diminutive size of the airway, craniofacial anomalies, laryngotracheal lesions, and acute (hyaline membrane disease, retained amniotic fluid) or chronic (bronchopulmonary dysplasia) disease. As these conditions are dynamic, they should be addressed pre-op-eratively in order to anticipate problems and minimize perioperative morbidity.
The infant
Neurosurgery in infants encompasses a myriad of acute and chronic conditions. Children in this age group can present with a wide variety of pathologies requiring surgical intervention including trauma, congenital abnormalities such as craniosynostosis, hydro-cephalus, intracranial tumours, intracranial vascular lesions and seizure disorders. Infants are prone to accidental as well as inflicted traumatic head injury and often require emergent craniotomies. Craniosynostosis surgery is frequently performed in this period in order to minimize restriction on brain growth. Given the compliance of the infant cranial vault, occulted slow-growing tumours will manifest as abnormal cranial enlargement.
Anatomy
Te cranial bones fuse at approximately 12-18 months. Tese sutures come together at the anterior and posterior fonta-nelles. Te posterior fontanelle closes first, usually by 1 or 2 months, or may already be closed at birth. Te anterior fontanelle usually closes later, usually between 9 m onths and 18 m onths.
The Monro-Kellie hypothesis states that the sum of all intracranial volumes is constant, which is certainly true for adult patients and informs neuroanaesthetic management. However, neonates and infants are an exception to this rule due to their open fontanelles and unfused cranial sutures. Given the nature of the infant’s fontanelles and unfused cranial sutures, the mass effect of a slow-growing tumour or insidious haemorrhage is often masked by a compensatory increase in intra-cranial volume accompanied by head growth. Tus, in infants where such processes are suspected, a component of the ongoing clinical assessment is usually serial measurements of cranial circumference. When intracranial pressure (ICP) increases occur slowly, open fontanelles and cranial sutures separate allowing the intracranial volume to increase. Terefore, the symptomatic manifestations of an expanding intracranial mass may be delayed. As a result, infants presenting with signs and symptoms of intracranial hypertension frequently have fairly advanced pathology. In this situation, the most obvious finding on physical examination may be an abnormally large head. However, rapid increases in cranial volume due to acute massive haemorrhage or acutely obstructed cerebrospinal fluid (CSF) flow cannot be attenuated by expansion of the immature cranial vault and frequently result in life-threatening intracranial hypertension. Once the fontanelles and sutures have fused, children have a lower intracranial compliance than adults. Contributory factors include a higher ratio of brain water content, less CSF volume and a higher ratio of brain content to intracranial capacity. Consequently, when similar relative increases in ICP occur, children may be at increased risk of herniation compared with adults. Tus, in children with fused sutures and closed fontanelles, signs and symptoms of intracranial hypertension must be assessed rapidly and addressed in order to avoid undesired outcomes .
Children over 2 years of age
Physiology
Age-dependent dif erences in cerebrovascular physiology have a significant impact on the perioperative management of neurosurgical patients. An understanding of these differences is the foundation of good paediatric neuroanaesthetic care. Cerebral blood flow is coupled tightly to cerebral metabolic rate for oxygen, and both increase proportionally immediately following birth. Wintermark and colleagues determined the effect of age on CBF. Using CT perfusion techniques, they reported that CBF peaked between 2 and 4 years and settled at 7-8 years. Tese changes mirror changes in neuroanatomical development. In healthy awake children, CBF is approximately 100 ml (100 g brain tissue)-1. Neonates and infants have a lower CBF than older children at approximately 40 ml (100 g)-1. In children, CBF can represent up to 25% of cardiac output. Te proportion of cardiac output devoted to the brain varies with age, with infants and children having a larger distribution. Te autoregulatory range of blood pressure in a normal term newborn lies between 20 and 60 mmHg, reflecting the relatively low cerebral metabolic requirements and vascular resistance of the perinatal period. Although children younger than 2 years of age have lower baseline mean arterial pressures, they have a lower autoregulatory reserve and can theoretically be at greater risk of cerebral ischaemia. Tese factors place the infant at risk for significant neurological insults during neurosurgical procedures compared with adults.
Table 14.1 Common perioperative concerns for infants and children with neurological problems
|
Condition |
Anaesthetic implications |
|
Denervation injuries |
Hyperkalemia after succinylcholine |
|
Resistance to non-depolarizing muscle relaxants |
|
|
Abnormal response to nerve stimulation |
|
|
Chronic anticonvulsant therapy |
Hepatic and haematological abnormalities |
|
Increased metabolism of anaesthetic agents |
|
|
Arteriovenous malformation |
Congestive heart failure |
|
Neuromuscular disease |
Malignant hyperthermia |
|
Respiratoryfailure |
|
|
Sudden cardiac death |
|
|
Chiari malformation |
Apnoea |
|
Aspiration pneumonia |
|
|
Hypothalamic/pituitary lesions |
Diabetes insipidus |
|
Hypothyroidism |
|
|
Adrenal insufficiency |