Introduction
To cover the brain’s metabolic demand, an adequate cerebral blood flow (CBF) is required. he brain is enclosed by the non-expandable skull and an increase in intracranial pressure (ICP) may reduce cerebral perfusion pressure (CPP), and impair CBF leading to cerebral ischaemia. Both CPP and ICP are established treatment targets in neurocritical care. Measurement of ICP has a high diagnostic and prognostic impact.
The Monro-Kelly doctrine
The brain weighs about 1400 g and occupies the majority of the intracranial compartment (83%). Cerebrospinal fluid (CSF) and cerebral blood volume (CBV) occupy approximately 11 and 6% of intra-cranial volume, respectively. We used to believe that the brain floats in the fluid, losing its relative weight according to Archimedes’ law. In fact, there is not enough volume of CSF to make such a mechanism effective. However, because of the continuous fluid environment, all gradients of the ICP within the CNS are equilibrated according to Pascal’s law. herefore, under normal conditions, there is no risk of volume shifts or herniations. his role of CSF is important for understanding why, following head injury, there is a critical threshold for increased ICP in the range of above 20-25 mmHg. Following head injury, due to brain swelling, the normal pathways of CSF circulation are usually obstructed and pressure gradients may develop. Meanwhile, in communicating hydrocephalus, during pressure-volume studies, patients tolerate rises in ICP of up to 40-50 mmHg, usually without any symptoms or adverse effects.
In 1824, Kelly outlined the fundamental principle later applied to our understanding of the dynamics of ICP, known as the ‘Monro-Kelly doctrine’ (Dr Monro was Dr Kelly’s teacher and at time of publication he was already deceased; the doctrine is a rare example in science of a pupil’s gratitude extending beyond the grave). It states that the brain is enclosed in a non-expandable case of bone and the brain parenchyma is nearly incompressible. Furthermore, the CBV in the cranial cavity is therefore nearly constant, and a continuous outflow of venous blood from the cranial cavity is required to make room for continuous incoming arterial blood. In other words, the volume of the intra-cranial compartment must remain constant if ICP is to remain constant. It is worthwhile remembering that in the original Monro-Kelly doctrine, the volume of CSF was not taken into account.
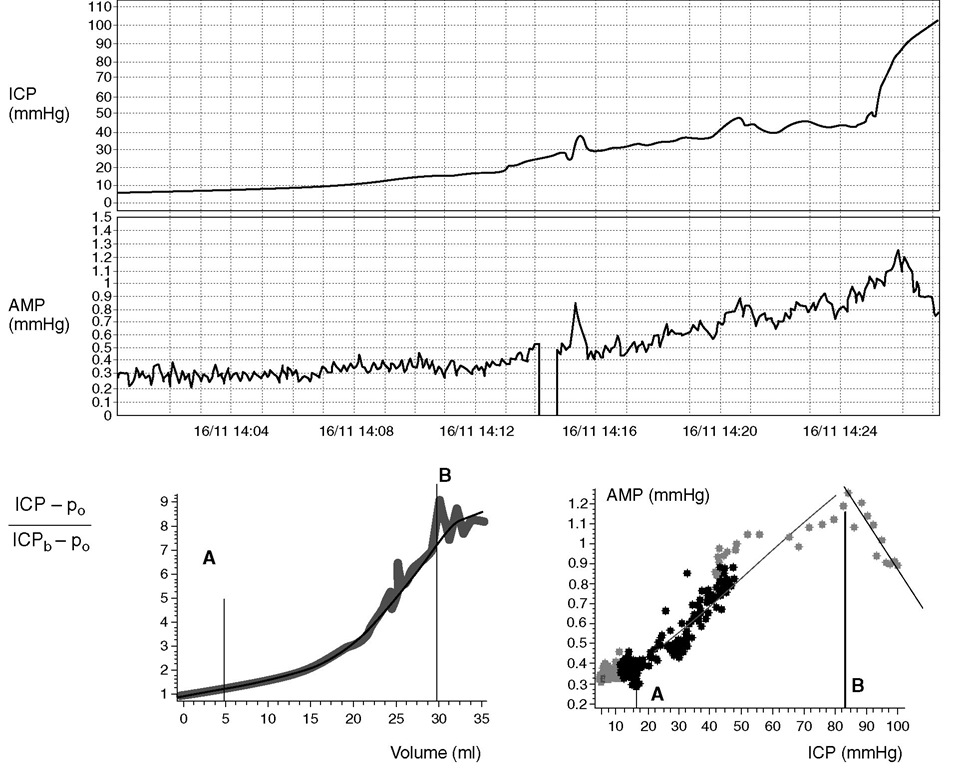
The cerebrospinal pressure-volume curve is nonlinear (Fig. 4.1). hree parts of the ICP-volume curve are described. he curve is flat at lower intracranial volumes, where good compensatory reserve is found. he ICP remains low and stable despite changes in intracra-nial volume. his is due to the compensatory mechanisms of the reduction of volume of CBV and CSF. he venous blood and CSF pools are considered to have the highest compliance of all compartments and are the first to be affected by raising ICP. However, when these compensatory reserves are reduced or exhausted, the curve rapidly turns exponentially upwards. his part of the curve represents a phase of low compensatory reserve, where ICP increases considerably, even with relatively small increases in intracranial volume. At the end, at high levels of ICP, the curve flattens again, CPP is very low and ICP approximates to the mean arterial blood pressure (ABP). A further increase in ICP leads to a collapse of the cerebral arterial bed if Cushing’s response is not able to keep CPP above the critical closing pressure of the cerebrovascular bed (20-30 mmHg).
Fig. 4.1. Top panels: Experimental intracranial hypertension provoked by saline infusion into the lumbar subarachnoid space. In response to an increase in mean intracranial pressure (ICP), the pulse amplitude of ICP (AMP) starts to rise, until a level just above 80 mmHg is reached, above which a decrease in AMP is observed. Bottom left panel: The net volume of increasing CSF (i.e. infusion plus presumed production of CSF minus reabsorption integrated in time) is plotted against the relative increase in ICP (ICPb, baseline pressure; po, reference pressure level), showing the full shape of the pressure-volume curve. Distinct regions are observed, delimited by the vertical lines A and B. Below A, pressure reacts weakly to changes of volume, indicating a good compensatory reserve. Between A and B, pressure increases exponentially with an increase in volume. Above B, the slope of the pressure-volume relationship decreases and finally saturates. This is a region of ‘extra compensatory reserve’, where the cerebral arterial bed collapses. Bottom right panel: The relationship between ICP and waveform amplitude provide a means of monitoring the physiology described above. Points A and B correspond to the lower and upper breakpoints of the relationship between AMP and mean ICP. Below A, the amplitude is constant; between A and B, it increases linearly with mean ICP, and above B, it decreases with a further increase in mean ICP.
Determinants of intracranial pressure
In most organs of the human body, the reference pressure for blood perfusion is either low or is coupled to atmospheric pressure. his does not apply to the intracranial space. he reference pressure (the outflow pressure) for brain perfusion is ICP, and in many pathological conditions it may be elevated. he ICP is derived from the circulation of cerebral blood and CSF. In the horizontal position, it may reach any value between right atrial pressure and ABP. In the vertical position, it usually decreases by ‘hydrostatic distance’ between the heart and the cranium. ‘Normal ICP’ in the horizontal position is from 5 to 15 mmHg. he limit of ‘abnormal pressure’ depends on pathology, and in head injury, ‘elevated ICP’ is said to exist at 20-25 mmHg.
Generally, the ICP has three components: (i) an arterial vascular component; (ii) a CSF circulatory component; and (iii) a venous outflow component.
The vascular component is difficult to express quantitatively. It is probably derived from the pulsation of the CBV (all waves, as heart rate-related changes in CBV and active vasogenic slow changes in CBV may play a role) and averaged by non-linear mechanisms of regulation of CBF. More generally, multiple variables such as the arterial pulsatile pressure, autoregulation and cerebral venous outflow all contribute to the vascular component.
The circulatory CSF component has been probably most widely investigated, and may be expressed using Davson’s equation:
CSF circulatory component of ICP = (resistance to CSF outflow) x (CSF formation)+ (pressure in sagittal sinus).
CSF is actively filtered from brain arterial blood, circulates along natural fluid passages and pools, and is absorbed into cerebral venous blood. Under normal conditions, without long-term fluctuations of the CBV, the production of CSF is balanced by its storage and reabsorption via the sagittal sinus:
Production of CSF = circulation of CSF = storing of CSF + reabsorption of CSF
The storage of CSF is one of the fundamental mechanisms assuring favourable mechanical conditions for the CNS. Circulation of CSF, if disturbed by closing anatomical channels for CSF flow, for example due to brain oedema, may produce acute intracranial hypertension. Reabsorption of CSF fluid into the venous compartment takes place predominantly (in humans) through arachnoid granulations that penetrate the walls of the sagittal sinus. It is important to recognize that reverse transport through the arachnoid granulations is impossible, i.e. drainage ceases if the subarachoid ICP is less than sagittal sinus pressure (SSP). An alternative component of CSF drainage (in pathology) is a possible leakage into the brain parenchyma. his can be visualized on CT or MRI scans as periventricular lucency, i.e regions of hypodensity, particularly around the horns of the lateral ventricles. Monitoring of a radioactive tracer injected into the lumbar space often shows that the tracer accumulates in ventricles. his is indirect evidence of intraparenchymal CSF absorption, consistent with the clinical picture of normal pressure hydrocephalus, both idiopathic and post-injury. Periventricular CSF is probably subsequently absorbed into capillaries or passes into perivascular spaces and is transported inversely to the direction of CBF outside the cranial cavity and absorbed in lymphatic nodes. Although this mechanism helps to reduce the CSF component of ICP, it may interfere with regional CBF and probably metabolic rate in the white matter.
In addition to the net circulation at a rate equivalent to its production, CSF is also subject to pulsatile fl ow. Pulsatile CSF fl ow is observed in the aqueduct cerebri (with a stroke volume of around 40 |fl) and in the cervical region of the subarachnoid space (with a stroke volume of around 500 ^l). For half of the cardiac cycle, fluid flows down into the spinal subarachnoid space, and for the remaining part it flows upward. Net aqueductal CSF flow is small in comparison with a pulsatile ‘void flow’ (it is equivalent to around 5 ^l in stroke volume, providing the heart rate is 60 strokes min-1). his movement of CSF is caused by pulsatile arterial blood inflow and venous outflow. Mismatch between infl ow and outflow of brain blood seen temporarily during one cardiac cycle is compensated by CSF movement. Pulsatile movement of CSF is undoubtedly associated with the pulse wave of CSF pressure, which is almost always present in recordings of ICP. he role of CSF fl ow and pressure pulsations is unclear but is being studied in hydrocephalus and other diseases that manifest with abnormal CSF dynamics.
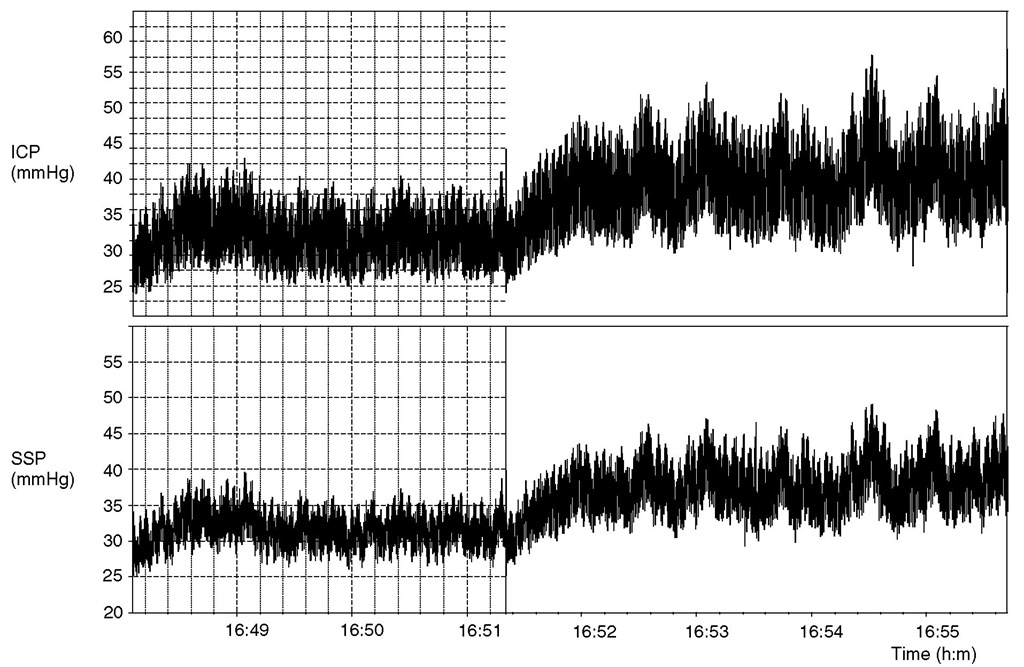
The last component of ICP – venous outflow – is sometimes neglected. In fact, any factor increasing central venous pressure also increases ICP. In addition, obstruction of venous outflow from the intracranial cavity (cerebral venous sinus thrombosis, or sinus collapse due to extrinsic pressure, etc.) may increase ICP. Coupling between the ICP and pressure in bridging and cortical veins provides the basis for the definition of CPP. Another coupling mechanism between ICP and venous outflow may be associated with dynamically collapsing venous sinuses. his mechanism has been observed in idiopathic intracranial hypertension, but it may provoke intracranial hypertension in other diseases. A rise in ICP squeezes sinuses, producing a rise in sinus pressure, obstruction of cranial venous outfl ow, further rises in ICP, etc., in a positive-feedback loop fashion (Fig. 4.2).
Any factor that disturbs CBF or CSF circulation may provoke an increase in ICP. his may occur in the absence of CNS pathology (e.g. with jugular compression during a Queckenstedt test or an endotracheal tube tie, or with very high intrathoracic pressures) or with intracranial pathology (e.g. brain swelling, space-occupying lesions or obstruction of CSF circulation pathways).
Fig. 4.2. An example of cerebrospinal fluid (CSF) pressure (intracranial pressure, ICP) and sagittal sinus pressure (SSP) recording in a patient with idiopathic intracranial hypertension. Both ICP and SSP were elevated (32 and 30 mmHg, respectively). An increase in ICP was caused by a constant rate (1 ml min-1) of infusion of Hartmann’s solution into the CSF space to evaluate resistance to CSF outflow. The observed rise in ICP was associated with an increase in SSP. During the infusion, slow waves in ICP and SSP were obviously associated.
Any increase in ICP may reduce CPP, reduce CBF and cause ischaemia. Ischaemia can cause a further rise in ICP due to increased cerebral oedema and further reduce CPP. his mechanism may develop into a vicious positive-feedback loop, causing irreversible brain damage. herefore, ICP is both an important surrogate marker of injured brain and a potential cause for secondary insults .
Intracranial compliance
Intracranial compliance is a concept often associated with CSF storage. It is defined as the change of pressure (dP) of per unit as change of volume (dV) per unit. he inverse of the compliance is called elastance (dP/dV), also known as the volume-pressure response (VPR).
Compliance decreases with increasing ICP, and the VPR or elastance increase with rising ICP. It has been demonstrated that the non-linear volume-pressure relationship could be modelled as a straight-line relationship to the logarithm of volume to pressure. his implies a monoexponential relationship between volume and pressure. his relationship has been described quantitatively by a pressure-volume index (PVI), which is the volume required to raise ICP tenfold. he PVI can be determined by rapid injection or withdrawal of fluid from the CSF space and measuring the pressure change.
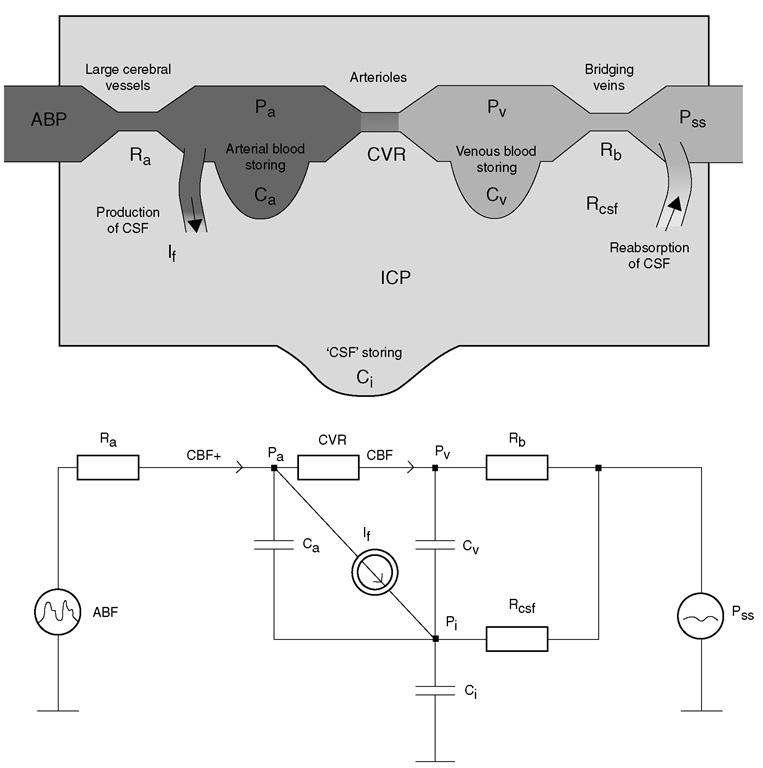
In fact, cerebrospinal compliance is more complex than a single lumped parameter. Physiologically, it can be expressed as the sum of three compliances: CSF space (associated with CSF buffering capacity), arterial bed compliance and venous compliance (Fig. 4.3). Arterial compliance is controlled by active modification of a tension of arterial wall smooth muscles. his compliance is, in most circumstances, the lowest of the three components. he venous compartment has far greater compliance, and cerebral venous blood volume is an important component of cerebral buffering capacity, as cerebral venous pressure is low and approximates ICP. his buffering volume does not represent a high proportion of total cranial volume, just around 70 ml, but after exhaustion of all CSF buffering capacity (150-170 ml), venous blood volume is the next buffer. If, after head injury, we cannot see any CSF volume in the ventricles and basal cisterns on a CT scan due to brain swelling, it is not unequivocally associated with exhaustion of venous blood buffering capacity and high ICP. A further decrease in the venous blood volume reserve may result in reductions in blood flow, a phenomenon that commonly occurs at a range of CPP below the lower limit of autoregulation of CBF.
Fig. 4.3. A lumped hydrodynamic model of cerebral blood flow (CBF) and cerebrospinal fluid (CSF) circulation (upper) and its electrical equivalent (lower). Arterial blood flow is into the intracerebral space through a linear low resistance, Ra. The volume of arterial blood is stored in arterial compliance, Ca. The main cerebral resistive vessels (CVR) are where autoregulation acts. The blood then flows to the venous part of the cerebral circulation and is stored in a venous and capillary pool (Cv). Rb represents the squeezable venous structures (cortical and bridging veins). The CSF is formed from arterial blood, and flows around all structures and is absorbed (through resistance Rcsf) back to the blood in the sagittal sinus (Pss). The compliance of the CSF space (C J is associated with the ability of the lumbar dural sac to expand in the lumbar canal through compression of the venous plexi. From the aspect of addition of CSF (point P , on the electrical diagram), all three compliances -arterial, venous and CSF – are connected in parallel; therefore, net intracerebral compliance can be approximated as Ca + Cv. + C , Pa,cerebral arterial blood pressure in small arteries; Pv, venous blood pressure; ICP, intracranial pressure; ABP, arterial blood pressure; If, CSF inflow.
In fact, following traumatic brain injury (TBI), all three compensatory mechanisms – autoregulation of CBF, CSF compensation and venous blood compensation – may be exhausted very dynamically, almost in parallel, allowing a very short time for clinical intervention before the development of refractory intracra-nial hypertension.
Measurement of brain compliance is classically performed using a CSF bolus injection. However, one recent ICP monitoring device includes a distensible balloon that can be repeatedly inflated for measurement of intracranial compliance. New and less invasive techniques for compliance estimation include phase-coded MRI (which is the most accurate) and transcranial Doppler ultrasound (TCD) (probably less accurate but suitable for continuous monitoring).