Clinical applications
Monitoring of cerebral oxygenation in critical care
Jugular venous saturation below 50% suggests a relative failure of oxygen supply compared with demand, and such episodes have been demonstrated to predict poor outcome after head injury in a dose-dependent way. Low SjO2 is often seen in comatose patients with head injuries or subarachnoid haemorrhage, even when therapy is guided by invasive haemodynamic and ICP monitoring. Jugular venous desaturation can be observed to occur before or without changes in measured CPP. Furthermore, desaturation events are particularly common in the first days after head injury and are usually secondary to poor perfusion secondary to hypotension or hypocapnic vasoconstriction, suggesting that it might be a meaningful parameter to use when targeting cardiovascular and respiratory support in the emergency setting.
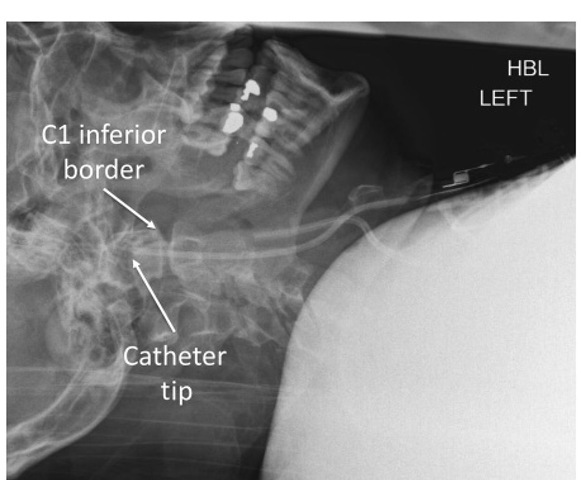
Fig. 6.4. Lateral radiograph of the cervical spine showing a jugular bulb catheter positioned with its tip cephalad to the inferior border of the first cervical (C1) vertebra.
Conversely, values of SjO2 >85% suggest a state of hyperaemia (for example secondary to hypercapnia) or failing oxygen utilization either due to a state of cellular dysoxia or due to shunting of arterial blood, which may also be associated with a poor outcome.
From the above, it would appear that jugular venous saturation measurement is a specific indicator of imbalance between oxygen supply and demand with at least prognostic significance. Unfortunately, false-positive desaturations are common. Moreover, it is by its very nature a global, hemispheric measure and thus may have poor sensitivity under certain circumstances. Focal areas of ischaemia are not reliably detected, SjO2 remaining normal until 13% of the brain becomes ischaemic. Indeed, regions of shunt from infarcted tissue may mask the effect of surrounding ischaemic areas leading to falsely reassuring values of SjO2.
Jugular bulb oximetry has been used to guide hyperventilation therapy in acute intracranial hypertension. Excessive hypocapnia is associated with a global reduction in CBF that may be harmful. Under such circumstances, cerebral oxygen supply falls and this should be detected as a fall in SjO2. However, a normal SjO 2 may be falsely reassuring, as regional hypoper-fusion may be present but not detected.
Jugular bulb oximetry can be used to detect disorders of both cerebral autoregulation and carbon dioxide reactivity. Assuming a constant CMRO2 , SjO2 is expected to rise in response to transient increases in cerebral perfusion. Fortune and colleagues studied a group of head-injured patients and identified instances where the rise in SjO2 due to hyperaemia persisted significantly beyond the perfusion returning to baseline, suggesting a disturbance of vasoconstriction in these cases. By measuring the response of AVDO2 to blood pressure manipulation and changes in arterial carbon dioxide tension (PaCO2), Sahuquillo and colleagues demonstrated that jugular venous oximetry could be used to differentiate between autoregulatory dysfunction and failed carbon dioxide reactivity, which may occur independently.
Failure of oxygen delivery, as evidenced by a fall in jugular venous oxygenation, is at best a measure of global tissue hypoxia. In failure of the aerobic metabolism, cerebral lactic acid production is increased, which can be used as an additional measure of cerebral oxy-genation. If blood is sampled from the jugular bulb, an arteriovenous lactate difference (AVDL) can be determined by comparison with arterial blood. he lactate oxygen index (LOI), which relates lactate production to oxygen extraction, is calculated as:
Patients with failing oxygen extraction typically have values of LOI >0.08. Cerebral lactate production is markedly increased in the first 24 h after head injury, with both AVDL and LOI having prognostic significance, even when oxygen extraction, derived from SaO2 and SjO2 measurements, remained apparently normal. It is noteworthy that the LOI implicitly depends on haemoglobin concentration (through AVDO2t , which may lead to falsely high values in anaemia.
Intraoperative monitoring
It has been shown that jugular venous desaturation occurs on average in 50% of patients undergoing a variety of neurosurgical procedures (with a higher incidence in patients undergoing aneurysm repair or with intracranial haematomas).
Table 6.1 Factors determining jugular venous oxygen saturation
|
Decreased SjO2 (relative hypoxia) |
Increased SjO2 (relative hyperaemia) |
|
Abnormal autoregulation |
Abnormal autoregulation |
|
Reduced oxygen delivery |
Increased oxygen delivery |
|
• Inadequate cerebral perfusion pressure |
• Increasing cerebral perfusion pressure |
|
• Vasoconstriction/hypocapnia |
• Vasodilation/hypercapnia |
|
• Vasospasm |
• Systemic hypertension |
|
• Arterial hypoxia |
• Arteriovenous malformations |
|
• Hypotension |
|
|
• Anaemia/haemoglobinopathy |
|
|
• Sepsis |
|
|
Increased oxygen consumption |
Reduced oxygen consumption |
|
• Increasing cerebral metabolism |
• Coma/sedative drugs |
|
• Hyperthermia |
• Hypothermia |
|
• Pain/inadequate analgesia |
• Cerebral infarction |
|
• Light anaesthesia/stimulation |
• Brain death |
|
• Seizures |
Cerebral oxygenation has been studied by jugular bulb cannulation during aneurysm clipping surgery. It was demonstrated that many patients exhibit a critical threshold for mean arterial pressure below which SjO2 falls. his was a more common finding in patients in which aneurysm rupture had occurred acutely, suggesting a state of altered autoregulation. However, a normal SjO 2 did not exclude an elevated LOI, as significant regional ischaemia may not have been detected. Furthermore, elevation of the LOI was found to be a predictor of poor short-term (although not long-term) outcome.
Cardiac surgery
Neurological injury is unfortunately not uncommon after cardiopulmonary bypass (CPB) and is believed to be a result of inadequate oxygen supply secondary to either microembolism or hypoperfusion. It seems reasonable to suppose that a measure of cerebral oxy-genation such as jugular bulb oximetry might be useful in the timely detection and hopefully prevention of damaging events. A correlation between jugular venous desaturation and poor post-operative cognitive outcome has been demonstrated, as has the fact that cerebral hypoperfusion that was diagnosed by oxim-etry could not have been detected by conventional intraoperative parameters alone.
It has been known for some time that jugular venous saturations are typically well maintained during hypothermic CPB, even in the presence of hypotension, as CMRO2 may be very much reduced compared with oxygen supply. Rewarming is, however, a much higher risk period for SjO2 desaturation, particularly when normothermia is rapidly restored, whereupon oxygen consumption rises quickly in the face of a potentially disturbed cerebral autoregulation mechanism. By comparison, oxygen extraction during normo-thermic CPB tends to be higher, with jugular venous desaturation episodes occurring soon after establishing bypass, reflecting the effects of haemodilution or hypotension, which may, to some extent, be ameliorated by careful anaesthetic technique.
Cardiac arrest
Jugular venous saturation has been studied as a potential prognostic marker in comatose patients in which spontaneous circulation has been restored after cardiac arrest (Table 6.1). Measured SjO2 is not normally low in these patients except under circumstances of cardiovascular failure when mixed venous oxygen saturation (SmvO2) also falls. However, in non-survivors, a gradual increase in SjO2 compared with SmvO2 is observed over the first 24 h after resuscitation, suggesting a progressive global failure of cerebral oxygen extraction. A SjO2 becoming greater than mixed venous oxygen saturation at 24 h appears to have reasonable specificity for mortality. A ratio of SjO2 to SmvO2 of >1 is similarly seen in brain death after severe head injury or intracranial haemorrhage.
Near-infrared spectroscopy
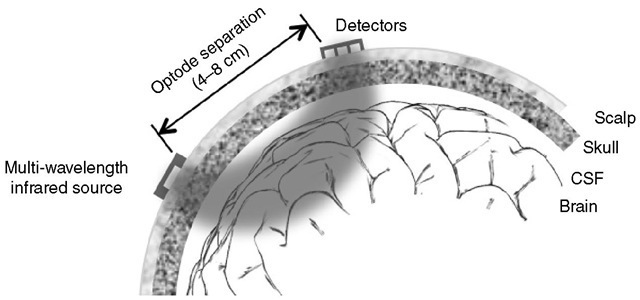
Biological tissues contain a number of light-absorbing pigments known as chromophores such as haemoglobin, myoglobin and cytochrome oxidase, which have absorption spectra that depend on their redox state. It so happens that these changes are reasonably pronounced in the near-infrared part of the electromagnetic spectrum (700-1000 nm) where absorption from skin and bone is low. Such light can thus penetrate several centimetres through scalp and skull to non-invasively probe brain tissue. By measuring optical absorption at several wavelengths, relative tissue chromophore concentrations can be inferred in real time and this forms the basis of near-infrared spectroscopy (NIRS) as a method for measuring regional cerebral oxygenation (Fig. 6.5).
Physical principles
The intensity of light passing through an ideal absorbing medium varies with the chromophore concentration and path length according to the well-known Beer-Lambert law. In principle then, it is possible to measure the concentration of a single chromophore from the optical absorption at a suitable wavelength. Where a mixture of different chromophores is present, it is necessary to perform absorption measurements at a number of different wavelengths. As the dominant cerebral chromophores, oxygenated and deoxygenated haemoglobin (HbO2 and HHb), have markedly different near-infrared absorption spectra, it is possible to infer the blood oxygenation status. As approximately 75% of cerebral blood is venous, NIRS oximetry gives a measure of predominantly venous oxygenation, which therefore reflects oxygen extraction.
In practice, the physics of light transport in biological tissue is more complex. Tissue is turbid so that light is attenuated not just by absorption but also by scattering. he Beer-Lambert law must be modified to include both absorption and scattering terms, as in the following equation, which complicates the interpretation of spectroscopic data:
where![]() is the source intensity, I is the detected light intensity, a is the absorption coefficient of the chromo-phore (a function of wavelength), c is the chromophore concentration, d is the distance between the source and the detector, B is the differential path length (discussed below) and G is a scattering term, which depends on optical geometry and tissue characteristics.
is the source intensity, I is the detected light intensity, a is the absorption coefficient of the chromo-phore (a function of wavelength), c is the chromophore concentration, d is the distance between the source and the detector, B is the differential path length (discussed below) and G is a scattering term, which depends on optical geometry and tissue characteristics.
Although the existence of scattering introduces additional complexity, it is useful clinically, as the adult cranium has a diameter too great even for infrared light to penetrate, making direct transillumination measurements impossible. However, by measuring the intensity of the back-scattered light instead, spectroscopy can be performed in a ‘reflection’ mode with the light source and detector (‘optodes’) applied to the scalp some 4-7 cm apart.
Multiply scattered photons do not travel directly from source to detector. Instead, they migrate randomly through an unknown volume of tissue before being detected, so that the actual path length is ill-defined. herefore, an average path length must be assumed that is rather longer than the optode separation. his effective path length is given by the source-detector separation multiplied by a scaling parameter called the differential path-length factor, which must previously be estimated from experiments.
Fig. 6.5. Schematic diagram of a typical near-infrared spectroscopy system. Two optodes are applied to the scalp away from large venous sinuses. The emitter can be switched sequentially between three or four infrared light sources of different wavelengths. Scattering causes light to diffuse randomly through scalp, skull, cerebrospinal fluid and brain tissues such that what arrives at the detector has passed through a ‘banana-shaped’ region. The penetration depth is proportional to the optode separation. The detector consists of an array of several photodiodes with different optode separations, an arrangement that allows the scattering contribution to be estimated.
Signal contamination by unwanted optical attenuation in extracerebral tissue is an important limitation of NIRS. Even if the optodes are positioned away from large venous sinuses, the light must necessarily traverse superficial tissue such as the scalp, whose blood content and oxygenation state are not necessarily constant and which may contain other oxygen-sensitive chromo-phores such as myoglobin. his may seriously affect the clinical reliability of the technique as a measure of cerebral oxygenation. Optical diffusion means that effectively a ‘banana-shaped’ volume between optodes is sampled, the depth of penetration being related to the optode separation. hus, if the source and detector are <4 cm apart, virtually only extracerebral tissue is sampled. Larger separations sample increasingly deep tissues, albeit at the expense of signal intensity and thus increasing noise. Even at 7 cm spacing, extracerebral contamination may be appreciable. By measuring at two different detector positions simultaneously, however, it is possible to at least approximately subtract the superficial tissue component.
It is difficult to measure absolute chromophore concentrations spectroscopically as the scattering term, G in the above equation, is unknown. However, if the scattering is constant then the above equation can be used to relate changes in absorption to corresponding changes in HbO2 and HHb (termed AHbO2 and AHHb, respectively) from an arbitrary baseline as the parameter G cancels out. his technique is known as differential NIRS.
More recently, a method known as spatially resolved NIRS has been described, which allows the absolute haemoglobin saturation to be measured. his technique employs a multiple-wavelength source and a probe containing several detectors at different path lengths. he scattering contribution is then estimated using photon diffusion theory. he derived ratio of HbO2 to total Hb (Hb + HbO2) is presented as a tissue oxygenation index (TOI) or regional oxygenation saturation (rSO2), which have similar utility.
The technique of NIRS can be used as a monitor of cerebral blood volume (CBV) as this is related to total haemoglobin concentration if the haematocrit is constant. Trends in CBV from an arbitrary baseline are then simply proportional to trends in total haemoglobin signal (AHbO2 + AHHb). Alternatively, spatially resolved NIRS can be used to calculate the tissue haemoglobin index (THI), which is a measure (in arbitrary units) of the total haemoglobin in the optically sampled region. Direct estimation of CBV itself is also possible by measuring AHbO2 and AHHb after an artificially induced small change in arterial saturation (for example, by varying the inspired oxygen concentration).
In addition to haemoglobin species, cytochrome oxidase has a near-infrared absorbance that changes with redox state. Cytochrome oxidase has a key role in transferring electrons to the terminal electron acceptor, oxygen, within the mitochondrial respiratory chain. Cytochrome oxidase comprises four metal centres (heme a, heme b, Cu. and Cub). he Cua centre is responsible for the infrared spectral characteristics of the molecule and is of particular interest. Its dimeric Cu-Cu form facilitates the transfer of electrons from cytochrome c to heme a. hus, the average Cua redox state reflects differences in the rates of electrons arriving and leaving. his may become perturbed if oxygen supply becomes metabolically limiting. Because changes in Cu. redox state result in changes in near-infrared absorption, in principle cytochrome oxidase NIRS offers a method for probing mitochondrial energy failure. Commercial instruments are now capable of measuring deviations of cytochrome oxidase concentrations from baseline, but such measurements remain controversial. Unfortunately, while the absorption spectra for HHb and HbO. are very different, reduction of cytochrome oxidase only influences absorption in a narrow absorption band. Additionally, the total contribution of Cua absorption to the total NIRS absorption is very small, making it difficult to separate from the haemoglobin signal.
![tmpA2-108_thumb[2] tmpA2-108_thumb[2]](http://what-when-how.com/wp-content/uploads/2012/04/tmpA2108_thumb2_thumb.jpg)