Hydrocephalus
Hydrocephalus comprises a miscellaneous group of disorders of CSF dynamics leading to an excessive accumulation of CSF within the brain, resulting in ventricular dilation and increased ICP. If hydrocephalus ensues before cranial sutures are fused, cranial fontanelles become tense and bulging, sutures are splayed and cranial circumference tend to increase abnormally.
It is of paramount important to stress the difference between the terms hydrocephalus and ventriculo-megaly. Tese terms should not be used interchangeably. Te term ‘ventriculomegaly’ describes the finding of ventricular enlargement on brain im aging and is not a specific feature of hydrocephalus. Ventriculomegaly is a common anatomical feature of brain atrophy following ischaemic and traumatic brain injury, or in senile dementia. In such cases, CSF compensates the intracranial space freed by a shrinking brain, causing the ventricles and cerebral sulci to appear enlarged.
Te CSF pressure is low and there is no indication for shunting. At the other end of the spectrum, patients with long-standing hydrocephalus and shunt obstruction can present with slit ventricles, despite impaired CSF reabsorption and increased ICP.
Impaired CSF reabsorption of dif erent aetiology is by far the most common cause of hydro-cephalus, while excessive CSF secretion caused by choroid plexus papillomas is a rare causal mechanism. Disturbed cerebral venous outflow is thought to play a significant role in the pathogenesis of idiopathic intracranial hypertension (IIH), and reduced brain compliance is probably involved in the pathogenesis of NPH.
Hydrocephalus can be categorized as congenital or acquired. Congenital hydrocephalus is present at birth and is often associated with developmental defects. It is typically non-communicating (obstructive) and requires urgent surgical treatment. Te incidence of infantile hydrocephalus is estimated at 1-5 cases per 1000 live births. Acquired hydrocephalus occurs after the development of the brain and ventricles. It is a more heterogeneous category, including obstructive and non-obstructive forms of hydrocephalus.
Pathogenetic classification subdivides hydroceph-alus into communicating and non-communicating forms. he term ‘communicating’ refers to the free flow and transmission of CSF pressure from the ventricles to the subarachnoid space. Non-communicating (obstructive) hydrocephalus is due to narrowing or obstruction of the normal CSF flow pathways within the brain. Non-communicating hydrocephalus causes a significant increase in ICP and usually has an acute presentation. It can be congenital (commonlyassociated with CNS malformations such as myelomeningocele) or acquired (caused by tumours compressing the aqueduct or intraventricular clots). Communicating forms of hydrocephalus have a much less intuitive pathogenesis. Communicating hydrocephalus is caused by ‘functional disorders’ of CSF reabsorption in the presence of patent CSF pathways. Communicating forms of hydro-cephalus, such as NPH, typically have an indolent and chronic clinical presentation. Normal pressure hydro-cephalus is a clinical syndrome presenting with the classic triad of gait difficulties, urinary incontinence and mental decline, as first described by Hakim and Adams in 1965. Although in NPH vasogenic CSF pressure waves can be prominent, baseline CSF pressure is usually normal, and – clinically – patients do not present with the typical signs and symptoms of increased ICP. he pathogenesis of communicating hydroceph-alus is a m atter of intense debate. he classical bulk flow theory suggests that communicating hydrocephalus is caused by a CSF reabsorption deficit at the arachnoid villi. More recently, hydrodynamic theories have been used to explain the features of communicating hydro-cephalus. According to the hydrodynamic concept, communicating hydrocephalus is caused by decreased cerebral compliance resulting in increased systolic pressure transmission into the brain parenchyma. he increased systolic pressure distends the brain towards the skull while simultaneously compressing the peri-ventricular region against the ventricles, which are fluid filled and therefore not compressible. Irrespective of the theoretical framework, ventricular shunting corrects the mechanistic CSF dynamics disorder of communicating hydrocephalus in both theoretical scenarios. On the other hand, the indication of a third ventriculostomy in communicating hydrocephalus has a rationale only in the context of the hydrodynamic theory.
Although not strictly classified as hydrocephalus, IIH is a disturbance of CSF dynamics that responds to ventricular or lumbar shunting, and is defined as a persistent increase in ICP in the absence of any intracra-nial lesions. he term IIH, introduced by Buchheit in 1969, corresponds to the former term ‘pseudotumour cerebri’; the term ‘benign intracranial hypertension’ should be avoided, as permanent visual defects are serious and not infrequent complications of this condition. Idiopathic intracranial hypertension is a relatively rare disease, but a rapidly increasing incidence is being reported due to a global increasing incidence of obesity.
Testing cerebrospinal fluid dynamics and shunt valve performance in vivo
Brain imaging can be misleading in the diagnosis of hydrocephalus. While the enlargement of cerebral ventricles is not necessarily of hydrocephalic nature – as in the aforementioned case of atrophic ventriculomeg-aly – even the finding of slit ventricles does not strictly exclude hydrocephalus and shunt malfunction.
Moreover, while shunting dramatically improves CSF dynamics, changes in ventricular size are often subtle or absent, even in shunt responders. In this context, it is often difficult to decide whether a patient needs a shunt or a shunt revision simply on the basis of brain imaging. However, it is possible to measure CSF outflow resistance and shunt valve performance with a simple bedside infusion test. Comprehensive computerized tools for evaluation of shunts are commercially available.
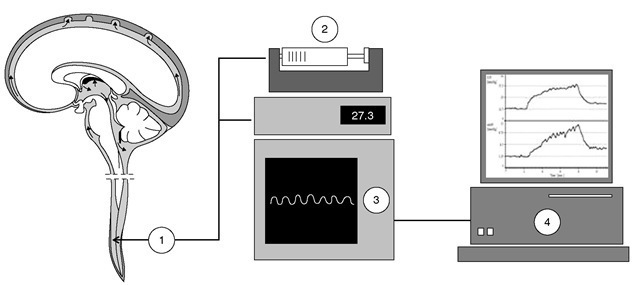
Computerized infusion tests can be used to estimate the resistance to CSF outflow (ROUT) pre-operatively (lumbar infusion studies, only in communicating hydrocephalus), intraoperatively (via ventricular reservoir, prior to shunting) and post-operatively (via shunt antechamber or ventricular reservoir). Pre-operatively or intraoperatively, the finding of increased resistance to CSF outflow (ROUT >13 mmHg ml-1 min-1) indicates a need for shunting. Post-operatively, the technique is used to detect shunt malfunction (including posture-related overdrainage and shunt obstruction) indicating a need for shunt revision. Infusion tests can be performed in awake or in anaesthetized patients (Fig. 17.2). In communicating hydrocephalus, access to the subarachnoid space is usually obtained via two lumbar needles that are connected to an infusion pump and to a pressure transducer via a stiff saline-filled tube. he CSF pressure, zeroed at the level of external acoustical meatus, yields a measure of ICP. In communicating hydrocephalus, the lumbar access is substituted by two 27G needles connected to the shunt antechamber or, intraoperatively, to the ventricular reservoir, providing a direct measure of ICP. Although infusion studies are listed as part of the management of hydro-cephalus by recent NPH guidelines, individualized CSF dynamics assessment is not yet common practice. Computerized CSF infusion studies provide a quantitative diagnosis of shunt malfunction, comparing the in vivo findings with the expected performance of the specific shunt model. Such tests can also be performed on outpatients, avoiding unnecessary admissions and significantly reducing the need for revisions.
Fig. 17.2. Infusion tests. In communicating hydrocephalus, access to the subarachnoid space is usually obtained via two lumbar needles (1) that are connected to an infusion pump (2) and to a pressure transducer (3) via a stiff saline-filled tube. The cerebrospinal fluid (CSF) pressure, zeroed at the level of external acoustical meatus, yields a measure of intracranial pressure (ICP). The response of ICP to the infusion of fluid can be plotted (4) to provide information about CSF dynamics. In communicating hydrocephalus, the lumbar access is substituted by two 27G needles connected to the shunt antechamber or, intraoperatively, to the ventricular reservoir, providing a direct measure of ICP.
Surgical technique and shunt valves
For ventricular shunts, the distal catheter is positioned in the peritoneum, right cardiac atrium or, less commonly, the pleura. For ventriculoperitoneal shunts, a high abdominal incision is made, and the distal catheter is tunnelled subcutaneously from the scalp to the peritoneal cavity using a long, hollow, metal rod (tunnelling device). For ventriculopleural catheters, a thoracic incision is made, and the distal catheter is tunnelled from the scalp to the pleural cavity. For ventricu-loatrial shunt systems, the route to the caval system is established by a lateral, right-sided neck dissection and isolation of the external jugular vein, in which the distal catheter is advanced into the superior vena cava.
Lumbar shunts are indicated in communicating hydrocephalus and IIH. For lumbar-peritoneal shunts, the distal catheter is threaded subcutaneously through the tunnelling device from the lumbar incision to the peritoneal cavity. For lumbar-pleural catheters, a thoracic incision is made, and the distal catheter is tunnelled from the lumbar wound to the pleural cavity.
Shunt valves can be ‘flow regulating’ or ‘pressure regulating. Flow-regulating shunt valves maintain a constant CSF drainage flow over a wide range of CSF pressures (e.g. constant drainage of 0.3-0.5 ml min-1 -approximating physiological CSF production rate – for CSF pressures of 5-30 mmHg). Pressure-regulating shunt valves open when the pressure difference across the valve exceeds a predefined value. More recently, programmable shunt valves allow the opening pressure to be modified non-invasively. Programmable shunt valves are ‘ball-on-spring’ valves, which can be programmed by adjusting the tension of the spring with a magnetic rotor. Te valves are programmed transcu-taneously with a special programming tool, which is a purpose-designed strong external magnet.
Shunt systems may be accessorized with CSF reservoirs and/or ‘gravitational’ devices. Reservoirs are small silicone devices connected to the ventricular catheter proximal to the shunt valve. Reservoirs can be palpated under the skin and provide transcutaneous access to the CSF using hypodermic needles, allowing CSF sampling for microbiology and CSF pressure monitoring.
Over drain age of CSF may cause disabling symptoms, including dizziness or visual disturbances, as the patient stands up. Gravitational devices are anti-siphoning devices that prevent overdrainage of CSF. Tey are a built-in feature in some of the most recent devices .
Anaesthetic management for shunt surgery
Pre-operative assessment includes the standard age-specific anaesthetic work-up and interview. Specific issues related to increased ICP, including decreased level of consciousness, increased risk of aspiration, dehydration and electrolyte impairment are discussed in detail elsewhere. Sedative pre-medication is best avoided.
Invasive blood pressure monitoring is usually not necessary. Induction may be undertaken using either intravenous agents or volatile agents. Rapid sequence induction can be indicated in patients with impaired consciousness or at risk of aspiration. Ketamine is best avoided for its detrimental effects on ICP.
Intubation should be undertaken using an armoured endotracheal tube as the patient positioning predisposes to kinking of standard tubes. Te patient is positioned supine for ventricular shunts and in the lateral recumbent position for lumbar shunts. A warming air blanket is used to maintain normother-mia. Anaesthesia is maintained with TIVA or a volatile agent, and ventilation is controlled to normocarbia.
Systemic antibiotics should be administered at induction to prevent shunt infection, regardless of the patient’s age and the type of shunt valve used. Further antibiotic prophylaxis following the first 24 h is not indicated.
The advancement of the tunnelling device under the skin is the most stimulating part of surgery. Preemptive infiltration of the skin with local anaesthetic may be used to minimize autonomic responses to the manoeuvre. In the case of pleural shunts, positive-pressure ventilation is discontinued for a few seconds, allowing for some degree of pneumothorax in order to facilitate the advancement of the intrapleural catheter. After satisfactory positioning of the catheter, local anaesthetic may be injected into the pleural space for post-operative analgesia. Valsalva manoeuvres and end-expiratory positive pressure is delivered to favour adequate re-expansion of the lung before closure of the chest wall. Post-operative pain for ventriculoperi-toneal and ventriculoatrial shunting procedures is mild to moderate, and satisfactory analgesia can usually be achieved with a combination of paracetamol and NSAIDs, with additional opiates ‘as required’ for breakthrough pain. In the case of pleural shunts, chest pain can be severe, and oral opiates are usually required in the first 48-72 h post-operatively.
Spinal anaesthesia, and sedation for MRI in patients with shunts
Patients with shunts frequently present for general anaesthesia for unrelated surgical conditions. Te most common anaesthetic dilemmas in these patients relate to spinal anaesthesia and sedation for MRI.
Although there is no formal contraindication to performing a spinal anaesthetic in patients with CSF shunts, many anaesthetists refrain from using a spinal technique in this population, mostly for concerns related to the risk of shunt contamination and CNS infection. However, the risk of infection following a spinal anaesthetic is of theoretical concern, with literature showing that the incidence of meningitis after spinal anaesthetic or lumbar puncture is the same as in the general population. Concern that dural CSF leakage following a spinal anaesthetic in patients with a ventricular shunt could compromise shunt function is also unfounded. If reservoirs or shunt antechambers are not readily accessible for CSF sampling in febrile shunted patients, lumbar punctures are performed as a routine investigation by neurosurgeons in this patient group without any subsequent adverse effect on shunt performance.
Te use of spinal anaesthesia has also been reported to be safe and effective for elective abdominal and perineal surgery in paediatric case series. Te use of strict aseptic technique and antibiotic prophylaxis in shunted patients seem to be adequate precautions to ensure uneventful spinal anaesthesia. Pre-operatively, signs of systemic infection should be excluded, and normal functioning of the ventriculoperitoneal shunt should be ascertained in consultation with a neurosurgeon.
As small magnets are used in all types of programmable shunt valves, the safety of MRI scanning is frequently raised for patients with ventriculoperitoneal shunts. Te main risks for a patient with an implanted programmable shunt are related to the resetting of the valve, heating and dislodgement of the implant during the imaging procedure.
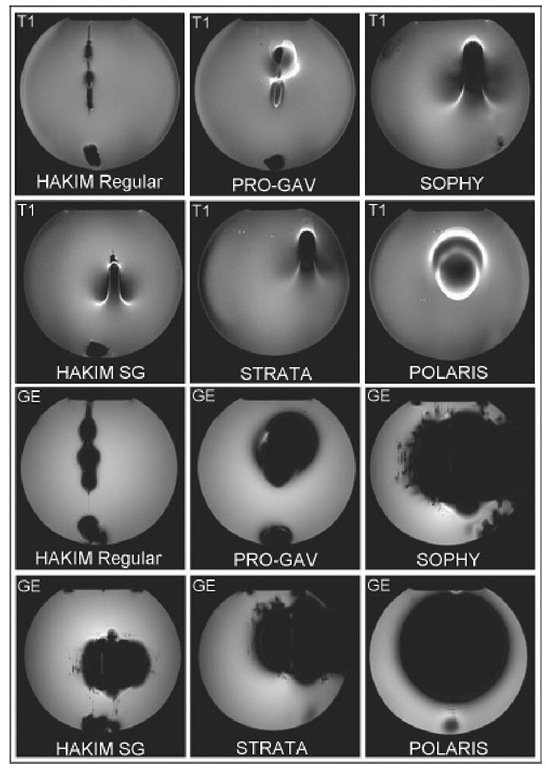
Fig. 17.3. Tesla MRI gradient echo (GE) andTI-weighted spin echo sequences (T1) revealing shunt valve-generated artefacts. The slice with the greatest artefact area is shown. Ghost diameter, 20 cm.
Moreover, shunt valves can generate considerable artefacts, thus invalidating the imaging procedure itself (Fig. 17.3). Imaging artefacts produced during MRI scans by programmable shunts vary and need to be checked on an individual basis, based on the shunt model and the diagnostic indication for imaging. Heating of the valves during MRI scanning is negligible for all commercially available models. Some models are resistant to inadvertent reprogramming during MRI, while others are promptly reset by exposure to weak magnetic fields. Checking the valve setting after MRI sequences should be considered a mandatory safety precaution in all patients carrying such shunts.
Anaesthesia for neuroendoscopic surgery
Aside from pituitary surgery, third ventriculos-tomy is the most widely performed neuroendoscopic procedure. Other conditions lend themselves to neuroendoscopic intervention. hese include biopsy or tumour retrieval, removal or fenestration of cysts, shunt placement and endoscopic strip craniectomy. he advantages of undertaking such procedures endo-scopically include accurate localization of intracranial lesions, especially when located in eloquent areas of the brain, access to regions not normally accessible by conventional surgery and reduction of surgical damage to healthy brain. here is also the potential to undertake tissue sampling prior to definitive treatment for a number of conditions. As there is minimal disruption to normal tissue, procedures tend to be shorter, with the possibility of early recovery and early hospital discharge.
Third ventriculostomy
Endoscopic neurosurgery was first performed by Lespinasse in 1910 when he used a cystoscope to coagulate the choroid plexus in a case of hydroceph-alus. hird ventriculostomy is commonly used to treat non-communicating hydrocephalus and is particularly used in patients with aqueduct stenosis, although a range of other conditions can also be managed this way. Contraindications to the procedure include abnormal ventricular anatomy, intraventricular haemorrhage and meningitis.
Hurd ventriculostomy involves accessing the horn of the lateral ventricle to undertake fenestration of the fl oor of the third ventricle. his creates a communication between the ventricular system and the basal cisterns, creating a path for CSF reabsorption that bypasses the aqueduct of Sylvius. A number of methods are used to create the hole, including using the scope itself, blunt probes or lasers. Once the hole has been formed, a balloon catheter is passed through the hole and expanded to dilate the opening. Maintaining CSF flow through the opening is fundamental to maintaining patency and therefore ensuring success of the procedure.
Procedure-specific complications include damage to the wall of the third ventricle (hypothalamus), which may occur if the procedure is undertaken in patients with smaller ventricles. A number of other critical structures are located in the vicinity of the ventricu-lostomy, most notably the basilar artery, as well as mid-brain structures. Cranial nerve palsies, SIADH, altered memory status and confusion have all been described. Venous bleeding is usually controlled with simple irrigation. Intraventricular clots may cause problems with CSF flow, necessitating further endoscopic diversion procedures. Te procedure itself requires the use of irrigation fluid, which in turn may cause acute rises in ICP. In small children in particular, the volumes of irrigation fluid used can also result in hypothermia unless adequately warmed. A range of arrhythmias has been described during these procedures including bradycardias and cardiac arrest. When successful, the need for an artificial shunt is eliminated, the success rate comparing favourably with that for shunts in children.