Chemical traces of memory and chemical sensitivity of memory
Many indirect observations illustrate the chemical nature of memory. For example, stability of memory after severe brain damage with catastrophic impairment of neural network structure demonstrates that a chemical hypothesis may be correct. When partial amnesic effects are ameliorated spontaneously or after remembering, this indicates to impairment of memory recall, while memory storage is preserved even during temporary amnesia. Where else, except in the chemical substratum, may temporary missed information be maintained? In addition, reorganization of neural pathways is a very long-lasting process when compared with chemical reorganization. It could be supposed that a copy of all supplies of long-term memory in each cell might be the safest way to protect behavior from brain damage. Remaining cells will possess all of the essential information required for survival. However, this guesswork is not proper.
Brain does recover its function after a long period of rehabilitation, days, weeks and months, but animals cannot to survive even a couple of weeks in the wild being completely helpless. Brain injury in ontogenesis and phylogenesis leads to weaker damage of behavior. Therefore, distributed properties of brain functions are not preferential in evolution and do not appear. In contrast, strict localization of function in brain structure increases in evolution, reaches a maximum in human and is completely absent in hydra, the most primitive multicellular animal [1255]. For example, complete extirpation of the neocortex renders a cat invalid, without any possibility for recovering, while in a turtle, extirpation of forebrain, together with the incipient cortex retains its viability. Habituation in the cold-blooded animals is observed even after decapitation. Localization of functions in the higher mammalian is not evidently an evolutional achievement. Behavior really recovers after brain damage, but rehabilitation of patients becomes possible only after the development of civilization. Capability of brain to recover its functionality is obviously explained by the ability of a brain part or even individual neurons to perform the functions of an entire brain. Rehabilitation is not, evidently, the result of successful brain construction, but rather the property of a neuron itself, which is simultaneously both the brain element and the living being. Of course, this idea may be right only if "cell consciousness" is rather primitive.
Experimental evidences exist verifying a close link between learning, memory and intracellular biochemical processes [1255, 663, 1039, 657]. In human, memory acquired in early childhood is fragile (infantile amnesia), but memory of the primitive animals much more stable. Their memory may stay intact while brain morphology is strongly reorganized in the course of individual development. During insect and amphibian metamorphosis, a subset of neurons within the central nervous system persists to participate in adult behavior, while neurons that will no longer be necessary die and some adult interneu-rons are born postembryonically. Hence, adult motoneurons, as well as some interneurons and modulatory neurons, are persistent cells, but neural networks undergo reorganization. In accordance with their new behavioral roles, these neurons undergo striking changes in dendritic morphology, intrinsic biophysical properties, and synaptic interactions [259]. However, a conditioned reflex acquired by a tadpole is sustained after metamorphosis [842]. Similarly, avoidance of a specific odor passes through stages of metamorphosis in fly [1276]. Conditioned odor avoidance produced in larvae still was present in adults 8 days later. Such memory through metamorphosis was specific to the temporal pairing of odor and shock. Presentations of odor alone or shock alone did not produce change. Part of larval neurons survive, some changing their peripheral targets and others innervating targets. Soma of neurons that persist during metamorphosis may serve as neuronal substrates of memory through metamorphosis. These facts are difficult to explain without an assumption of the chemical substrate of memory. After amnesia, long-term memory does not disappear: information can be recovered by means of reminder or by influence of some drugs [1255, 1039, 657]. Chemical alterations during learning have been discovered in many brain areas, but after the firm fixation of information, chemical traces of memory are concentrated in specific areas of the brain6. Damage of these specific brain areas together with their traces of memory does not lead to disappearance of acquired information. Evidently, memory traces in other brain areas from early stages of learning do not disappear absolutely, although they are not usually accessible.
Thus, learning leaves chemical traces in the brain. Moreover, chemical impact leads to alteration in memory. Fear conditioning is disrupted by inhibition of cyclic AMP-dependent kinase [86]. The second messenger cyclic AMP has a very fast and profound effect on many neuronal genes. Molecular genetic investigations of Drosophila olfactory learning have uncovered numerous genes whose gene products are essential for memory formation. Genes that impair olfactory memory when they are disrupted: adenylyl cyclase (that produces cyclic AMP), phosphodiesterase (that degrades cyclic AMP), protein kinase A (that leads to protein phosphorylation), etc. These genes have a prominent role in mammalian and non-mammalian organisms [298]. Reactivation of NMDA receptors is also necessary for the long-term storage of old memories in neural circuits.
Animal behavior and including learning change also after alterations in neurotransmitter systems of brain [680, 53, 481], after administration of neu-ropeptides [983, 314], after the addition of antibodies to many brain antigens [603, 973], after blockage of cytoplasmic flow [397] and as the result of activation of immune processes [62]. Single gene modification in Drosophila flies determines agressive behavior [1310]. Both genetic and immunologic memories depend on protein synthesis. Therefore, it was natural to suppose that neu-ronal memory is also associated with a similar mechanism and that acquisition of new memory is combined with the synthesis of new proteins. This parallel, however, is superficial, since neuronal memory is dynamic and established rapidly, when compared with genetic and immunologic memory.
There are some general molecular events related to memory storage that have been, in particular, identified in Aplysia sensory neurons, chicken fore-brain and mouse pyramidal neurons of hippocampus [86]. These are: equilibrium between kinase and phosphatase activities at the synapse, retrograde transport from the synapse to the nucleus, activation of nuclear transcription factors, activity-dependent induction of gene expression and synaptic capture of newly synthesized gene products. The location of these events, supposedly, moves from the synapse to the nucleus and then back to the synapse. Following transcriptional activation, newly synthesized gene products have to be delivered specifically to the synapses whose activation originally triggered the wave of gene expression. That is, hypothetically, the products of gene expression are delivered throughout the cell but are functionally incorporated only in those synapses that have been tagged by previous synaptic activity [86]. This description looks like a version of the chemical mechanism of memory, but it cannot explain the correspondence between chemical and informational processes and factually is in accordance with the synaptic plasticity hypothesis and may plausibly explain development of new pathways in ontogenesis, during damage, etc. Participation of the discussed chemical processes may be reduced to servicing. Transport from the synapse to the nucleus is non-specific and it cannot deliver a message to precise sites in the nucleus. A specific message cannot be transmitted by means of diffusion of unspecific substance. This is possible only through specific channels. For example, newly synthesized proteins have to be selectively transported to activated synapses without altering the function of all other synapses in the activated cell (hours and days) [1316]. A cell must somehow integrate signals from a pattern of synapses and translate information into chemical language. All aforementioned facts testify to the availability of chemical alterations in the brain during learning. However, the data add little to comprehension of chemical coding of information.
There is agreement between physiologists, pharmacologists and psychologists about the existence of at least two phases of memory that proceed through temporally distinct phases: short-term (minutes – hour) and long-term (hours days years) [1039]. Disruption of one does not disrupt the other. Sometimes ones distinguish instantaneous (seconds) and intermittent (hours) phases of memory. Instantaneous memory maintains traces of current signals. Phases of memory have specific sensitivity to influence different substances. Various phases differ by their steadiness and stability to amnesic impact. Long-term memory is considered to be the most firm and is very resistant to hurt. Memory transformation into a long-term phase is called consolidation of memory. In a simple form, consolidation hypothesis postulates existence of only two sorts of memory storage, each having a specific chemical characteristic and a different dependence on pharmacological impact, short-term and long-term memory [1039]. The cell nucleus is considered to be a participant in long-term memory, while short-term memory operates without nuclear application [86].
The most important property of long-term memory consolidation is its dependence on protein synthesis and on synthesis of ribonucleic acid (RNA) [477, 397, 1040, 20, 86]. Indeed, formation of long-term memory is impossible after blockage of protein synthesis, at least for stressed learning or learning with negative reinforcement. An overwhelming majority of data was achieved for specific, negative forms of learning and very rarely normal appetitive learning may depend on new proteins. In special circumstances, it was reported, instrumental learning with feeding reinforcement, also may depend on protein synthesis. So, early consolidation of instrumental learning requires protein synthesis in the nucleus accumbens [536] and strong inhibition of protein synthesis in the motor cortex (for 4 days) by means of anisomycin retarded appetitive learning [768]. Protein synthesis in experiments is usually blocked by antibiotics, but to our knowledge, nobody has observed amnesia in humans after antibiotic injections. In animal experiments typically anisomycin or elec-troshock are used in order to inhibit protein synthesis. On the other hand, anisomycin, which disrupts traumatic memory consolidation, inhibits anxietylike behavior [250]. Anisomycin can stimulate stress-activated protein kinases, and after anisomycin administration an animal behavior may look forgetful, but an animal may simply not execute a negative habit because its negativity was weaker after injection. Besides, anisomycin was described as downregu-lating gap-junctional intercellular communication [905] and this may prevent normal behavior. Blockage of protein synthesis leads also to enhancement of amino acid concentrations, because amino acids cannot be included in the protein structures [1040]. Many amino acids (especially glutamate and GABA) are neurotransmitters and affect the process of learning. A comprehensive review of the protein synthesis literature leads to conclusion that long-lasting memory is observed despite protein synthesis inhibition [1049].Therefore, the theory that memory consolidation is accomplished through macromolecule synthesis is premature.
Recently, a newly described phenomenon, memory reconsolidation, had been reported to occur after negative learning. During learning, punishment was presented immediately after a conditioned stimulus. Later representation of the CS+ alone evoked fear and the reaction of avoidance was accomplished and the animal escaped punishment. The phenomenon of reconsolidation was revealed by means of post-training with an intra-amygdala infusion of the protein synthesis inhibitor anisomycin. Amygdala, supposedly, is involved in modulation of memory for aversively motivated behavior. Anisomycin produced amnesia of the past memory if administered immediately after retrieval, that is, in the time period when consolidation had already been completed [880]. After retrieval, anisomycin leads to disturbance of the habit in the next trial, that is, next CS+ does not evoke avoidance, although without anisomycin avoidance would be still robust. This looks like forgetting the habit. The phenomenon seems to be that consolidated memory becomes labile again after retrieval and reconsolidation of the stable memory after retrieval requires protein synthesis.
Reconsolidation is an extremely firm hypothesis, since this is an energy-and substance-consuming process. Of course, each particular image recollection may correct memory, if it is enriched by new details. Fact recollection itself also may leave a trace in memory. Nevertheless, uninterrupted memory reconsolidation through an entire life, in a body, looks improbable. All the more, the aforesaid data are easy to explain without such a strong suggestion. We may suggest a more parsimonious explanation. Absence of a US leads to extinction and the next presentation of the CS+ may decrease instrumental reaction and increase probability appearance of the US. This standard scheme of extinction works properly for an appetitive conditional reflex because the animal immediately discovers the absence of a US. During aversive conditioning, abolishment of a US will for a certain period remain unknown to the animal: it is unclear, to the animal why the US is absent, because after it has accomplished the correct instrumental reaction or because of change in environment and abolishment of punishment at all. As a result, extinction of aversive habits is delayed. When the animal perceives the CS and accomplishes avoidance reaction, it does not receive punishment, but does feel fear. He is afraid the punishment and therefore escape it. Since anisomycin inhibits anxiety-like behavior [250], animal later remember the fact receiving of the CS and the fact of absence of fear. This must lead to reconsideration of the degree of danger. In the next test, the CS+ will be less connected with fear, animal will not perform the reaction of avoidance and this will be look like amnesia. Essentially that anisimycin-induced anxiety-like behavior tightly interacts in time with the situational reminder. Microinjection of anisomycin, administered 1 hour after the reminder, did not affect the anxiety-like behavioral response 3 days later [250]. Similarly, when electroshock, which is distressed closely to time of aversive learning trial, evokes amnesia, this may be connected with remembering stronger aversive event comparing with the weaker negative US. Therefore reconsolidation may be epiphenomenon. All the studies devoted to reconsolidation are based on punishment or aversive training and "reconsoli-dation" was detected only in the absence of significant extinction [341]. As we have explained, anisomycin may assist to display extinction. In particular, the dependence of "reconsolidation" on protein synthesis decreases with the age of memory [616]. Phenomena similar to "reconsolidation" were observed also after administration of drugs, which do not affect protein synthesis directly. These are opioid peptides, GABA, norepinephrine, cyclic AMP, acetylcholine and glutamate [821, 616].
The border between short-term and long-term memories is vague. The duration of memory components can vary with different drugs, tasks, reinforcement and species [316]. Spontaneous recovery or reminders-induced recovery of memory over a time course of weeks or months has been reported in animals which were initially thought to be amnesic [871, 1316]. Memory for a well-learned instrumental response does not require protein synthesis-dependent reconsolidation as a means of long-term maintenance [537]. Short intervals for consolidation also were demonstrated. During classical conditioning of negative reinforcement in earthworms (the occurrence of a shrinking response), inhibition of mRNA synthesis by actinomycin-D or protein synthesis by ani-somycin blocked consolidation of the long-term memory, when either of these two compounds was injected into the body cavity of the worm within 25 min of conditioning [1324]. Amnesia usually, with the rare exceptions, more concerns to recent events. Sometimes these are years, sometimes minutes. This means that consolidation of memory either continues uncertainly long or amnesic agents could affect the recall of memory instead of memory storage.
There are many doubts that memory is coded in the protein structures. Previously, the data concerning memory transfer from one brain to another by chemical substrates was seriously discussed, but now this idea is only of historical interest. J.V. McConnell [816] described memory transfer through cannibalism in planaria. Also G. Ungar [1286] and some others reported memory transfer in rats. If memory transfer would be confirmed by subsequent investigations, the suggestion of chemical coding of memory would be irrefutable. Memory transmission through metamorphosis and through heredity also is in agreement with the reality of a chemical code for acquired memory. Existence of the brain-blood-barrier may be interpreted, as the defense of newly synthesized brain antigens from immunological attack of the organism and this indirectly testifies in favor of the chemical nature of memory. However, consensus deems it unfeasible to transfer memory acquired during learning from brain to brain [194, 1040]. The effects that had been observed appeared as the result of such accompanying circumstances, as an augmentation of motor activity, induction of stress or depression, administration of endogenous opiates, etc. This, however, does not concern inborn behavior. Some general predispositions to an inborn form of specific behavior do exist and we will discuss this problem later. The possibility of behavior transfer using chemicals is indirectly supported by the numerous data involving the induction of motivational behavior by means of chemical substances. This is related to sexual behavior, feeding, drinking, etc. [1257]. State-dependent learning is another example of selective chemical influences to memory reproduction: some pharmacological modulations (for example: cholinergic substances) act as a specific chemical environment, and memory recall is possible only on the same chemical background, which takes place during learning [53]. Nonetheless, this is unlikely to assume transfer of ordinary habits between brains. Moreover, transmission of chemical memory between different regions of the same brain is also doubtful. For example, lobotomy as a form of psychosurgery, consisting of cutting the connections to and from the prefrontal cortex, results in major personality changes and these changes are irreversible [1294, 584], although the pathway for transmission of chemical information stays intact. Similarly, when the corpus callosum connecting the two halves of the brain is severed, we have the phenomenon of the split-brain and the patient perceives separately the data acquired from the left and right sides. This impairment also does not recover.
The surgical operation to produce this condition is rarely performed, usually in the case of epilepsy, and this operation reduces the severity and violence of epileptic seizures. Hence, even if chemical memory exists it is not a material substance observable regardless of neuronal structure, as, say, a hard disc containing information independent of a computer.
Biological meaning during habituation is acquired or lost by chemical means
Any brain activity is based on transformation between chemical and electrical processes. Only at the final stage does a nervous process convert to mechanical movement. A decision to generate or fail to generate a spike may be related to changes in synaptic efficacy and excitability and both changes depend on chemical reactions within neurons [827, 20]. Therefore, chemical processes do participate in learning and memory. Really, supporters of the memory hypothesis as the reorganization of a neural network (and even uttermost form of this hypothesis: memory in presynapses), fairly do not consider contradictions between synaptic and chemical memory [86]. Therefore, chemical processes certainly participate in memory and even if they have ancillary, non-informational functions, they will be augmented during learning and their blockage will interrupt memory consolidation. These processes may be associated with augmentation or decline of general metabolism or else certain informational metabolic pathways. However, there is a question, to what extent these chemical reactions participate in memory. They may provide energetic processes during learning [31], morphologic reorganization [77] and homeostatic recovery [478] that is, do not participate in the control of informational significance. When chemical processes have non-informational functions, the role of chemical reactions is reduced to ensuring the presence of memory traces in the space of a neural network.
On the other hand, chemical alterations during learning could perform informational loading and there has to be a parallel between elements of information and chemical processes or substances. During learning, responses to biologically important stimuli usually increase and responses to insignificant stimuli decrease. When neurons decide which stimulus is more important in the current behavior, is the decision made by chemical means? As a maximal presumption, different perceptual signals give rise to different chemical processes in the neurons. As a minimal presumption, chemical specificity may correspond only to a difference between signals, participating in current behavior and other signals. Let us consider how neurons distinguish a biological important signal from insignificant ones.
Learning depends on various chemical processes within neurons, but it is unclear whether these chemical processes have any informational significance. We tried to examine to what extent the signals having different biological significance may have a different chemical basis [1264]. If they did, injection in a neuron vicinity of the same biologically active substance before and after learning would have different effects on the response of the neuron. As a simple example of learning, we chose habituation in a mollusk and studied whether a change in reactions of an identified mollusk neuron for defensive closure of the pneumostome during elaboration of the neuronal analog of ha-bituation is connected with modification of the chemical processes induced in the neuron by a habitual (tactile) stimulus and whether this process differs from chemical processes induced by a new stimuli (light). Let us suppose that the chemical processes evoked within the neuron by the stimulus are modified during its repeated presentation, when the biological importance of a novelty is reduced and response to the stimulus decreases correspondingly. Then, microiontophoresis of the same substance before and after habituation would have different effects on the response of the neuron, if, of course, this substance somehow interacts with the learning-related chemical processes. As an example of a biologically active substance we used acetylcholine, which exerts modulatory action by means of muscarinic receptors [676, 1036]. Acetylcholine is readily availably to the learning process, is not a toxic agent and is naturally presented in neural tissue [183, 366, 481]. For instance, when in the rat somatosensory ‘barrel’ cortex the temporal frequency of whisker deflection, were modified by cellular conditioning, administration of acetylcholine during testing revealed frequency-specific changes in response that were not expressed when tested without acetylcholine [1140]. Acetylcholine also modulates an action potential waveform and thus connects synaptic and membrane processes [386].
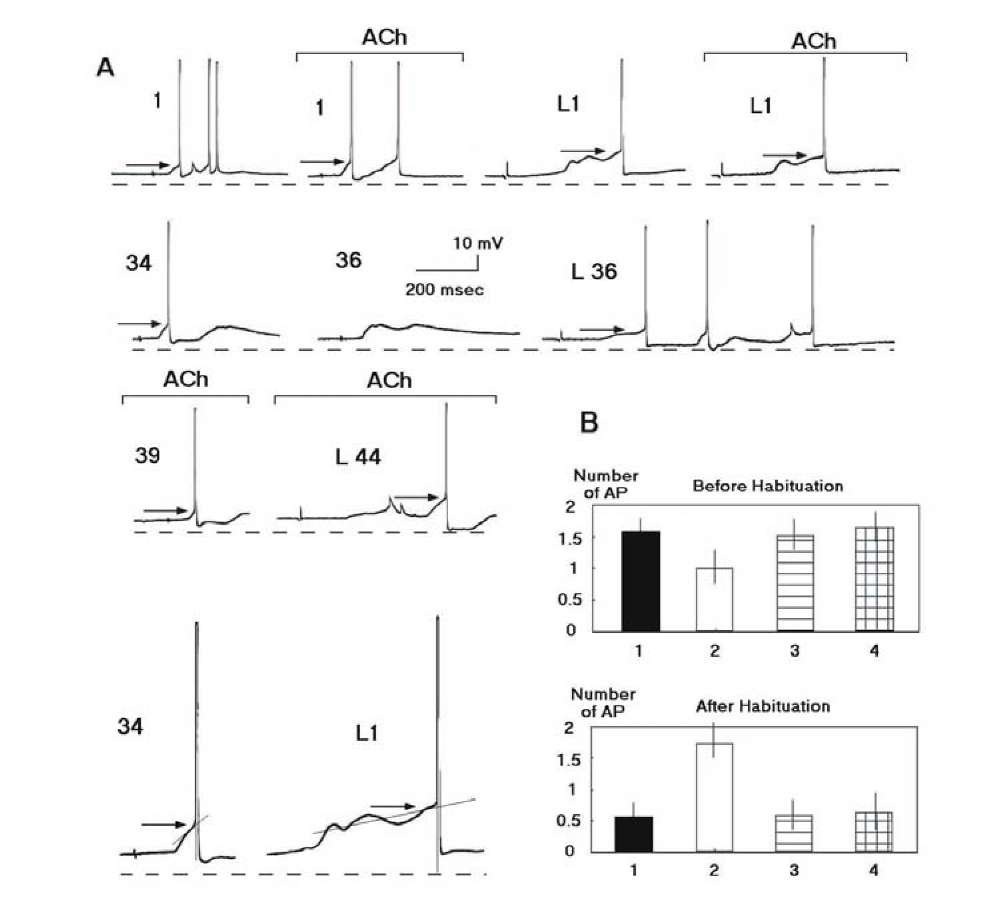
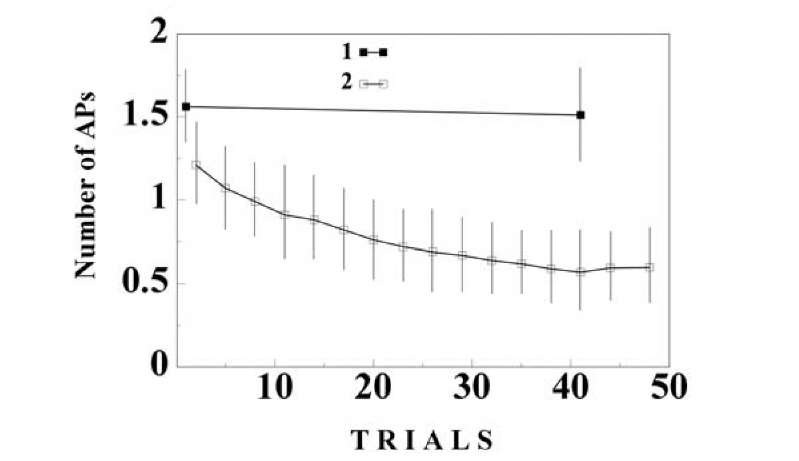
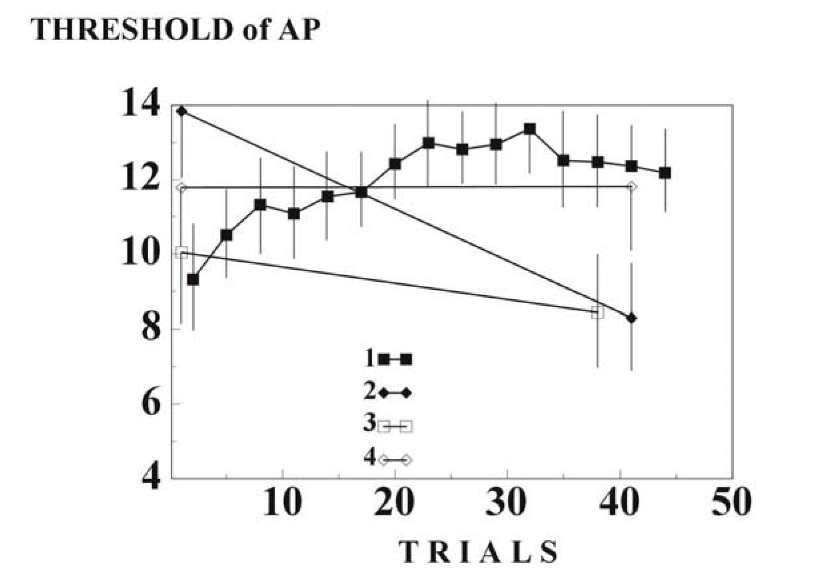
Habituation in our experiments was accompanied by a decrease in the number of action potentials in the response and by elevation of the threshold of AP generation, but was not accompanied by any appreciable change in membrane potential. The number of APs in response to switching off the light in the experimental camera increased after habituation and the threshold of the first AP in this response fell below the control level7 (Fig. 1.26A). This is in agreement with previously discussed data that a neuron may have different excitability corresponding to different signals.
The modulatory effect of acetylcholine in our experiments was exhibited almost in the absence of any direct shift of membrane potential under the influence of acetylcholine, which is in accord with previously published data [272, 355]. The influence of acetylcholine to the responses was blocked by atropine (Fig. 1.26B), in agreement with the data that indirect action of acetyl-choline to synaptic responses is insured by the muscarinic receptors. Although atropine prevents the modulatory action of acetylcholine, it did not exert any influence on responses evoked by the tactile stimulus and light (Fig. 1.26B), either before or after habituation. Postsynaptic sensitivity to acetylcholine did not change after habituation. Those experiments (9 of 34 experiments) where acetylcholine displayed direct excitatory action to a recorded neuron (1-4 mV depolarization), modulatory action of acetylcholine was not significant. This is in agreement with properties of neurons in the rat somatosensory system induced by a sensorysensory association [303].
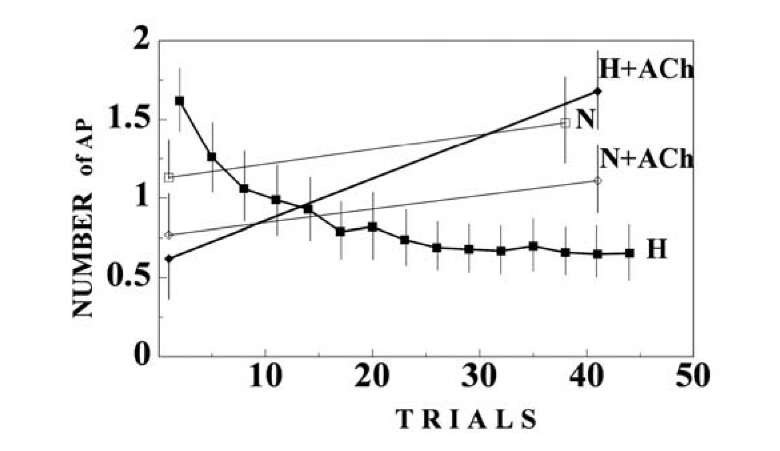
The number of APs in response to a tactile stimulus slightly decreased on the background of acetylcholine before habituation, but sharply increased after inhibition of the response during habituation (Fig. 1.27). Disinhibitory action of acetylcholine to frequent stimulus was found also in the rat’s hippocampus [676].
Qualitatively similar results were received in the control series when we used the tactile stimulus on the background of acetylcholine as a repeated stimulus, while the same stimulus without acetylcholine administration served as a rare stimulus (Fig. 1.28).
Changes in the number of APs, at least partially, may be explained by changes in excitability (Fig. 1.29). Acetylcholine, it is known, may play an important role in the regulation of neuronal excitability [272, 355]. Before habituation, the thresholds of AP generation in response to light and tactile stimuli changed weakly after administration of acetylcholine. After ha-bituation, introduction of acetylcholine disinhibited response to the habitual stimulus but raised the threshold of the first AP in response to light.
The effects of acetylcholine on afferent response properties could not be predicted from its ability to excite a cell. This suggests that the mechanism
Fig. 1.26. Cholinergic influences to the neuronal activities before and after elaboration of the neuronal analog of a habituation to repeated presentation of the stimulus. A – Representative intracellular recording of the activities of the identified neuron (Lpa3) during habituation. The numbers of the habitual stimuli (tactile: 1, 34, 36, 39) and the number of those habitual stimuli, which preceded the rare stimulus (light: L1, L36, L44), are indicated. Responses on the background of acetylcholine (ACh) are indicated by the line (current – 90 nA). Time interval between the responses in the first line (initial responses) was 5 min. Mean level of the membrane potential in the neuron was -61.2 mV. Dotted line indicates the level 65 mV. The arrows indicate thresholds. Responses 34 (simple case) and L1 (complex case) are magnified (X 2) and method of the threshold determination is presented (see paragraph 1.7). For the complex case, the tangent intersects the voltage trajectory near to the proposed point of inflection by as much as 5 points. Calibration, 10 mV, 200 ms. B A muscarinic nature of the cholinergic influences to the responses before (at the top) and after habituation (at the bottom). Ordinate: number of APs in the responses. Abscissa: 1 mean response to the tactile stimulus; 2 mean response to the tactile stimulus on the background of acetylcholine; 3 – mean response to the tactile stimulus on the background of muscarinic antagonist atropine; 4 – mean response to the tactile stimulus on the background of atropine and acetylcholine.
Fig. 1.27. Neuron acquires chemical specificity during habituation. The symbols are denoted in the figure. 1 – Responses to the repeated presentation of the tactile stimulus. 2 – Responses to switching off the light. 3 – Responses to the tactile stimulus on the background of acetylcholine. 4 – Responses to switching off the light in the background of acetylcholine. (C,D). Ordinate – the number of APs in the responses. Abscissa the trial number. The mean values and confidence intervals (p < 0.05) in the plots (A-D) are calculated by means of a two-way ANOVA with interactions between the trial number and the type of stimulus habitual or rare stimulus.
Fig. 1.28. Behavior of neuronal activity during habituation, the results of the control experiment. Repeated presentation of the tactile stimulus in the background of acetylcholine was used as the habitual stimulus, and tactile stimulus without microiontoforesis of acetylcholine was used as a rare stimulus. Abscissa the trial number. The symbols as in the figure 1-27.
Fig. 1.29. Chemical source of change in AP threshold during habituation. Ordinate, the threshold (mV) of the first AP in the responses. The symbols is in Fig. 1.26
Thus, we have found that the same substance (acetylcholine) in the same identified mollusk neuron affected responses evoked by the same tactile stimulus differently before and after habituation. We may conclude that the chemical process evoked within a neuron by the stimulus is rebuilt after habituation. Moreover, the same substance in the same identified mollusk neuron and in the same time (after habituation) affected differently to responses evoked by habitual and rare stimuli. Evidently, the chemical processes within the neuron evoked by the habitual and novel stimuli are different. Atropine blocked this specific action of acetylcholine, in agreement with data that the muscarinic cholinoreceptors play a role in neuronal plasticity. We suppose that the chemical processes discussed are within the recorded neuron, because of relatively local action of the acetylcholine in the vicinity of the neuron soma and because a threshold is the intra-neuronal property of an excitable membrane. This experiment does not reveal the peculiarity of chemical reactions, but it does prove specific chemical changes during learning.
ACh in our experiments helped to reveal the properties of a chemical process evoked within the neuron by the given stimulus. This phenomenon may be related to another well-known property of ACh. A number of choliner-gic substances are capable of producing state-dependent learning [877, 53], in which an animal retrieves the newly acquired information only if this animal is in the same physiological state as it was during the acquisition phase. In particular, habitual stimulus in a new chemical environment may be perceived as a new stimulus. It has been shown that ACh can induce state-dependent learning at the cellular level [1140]. However, state-dependent learning, which evidently might take place in our experiments, is not an alternative explanation of our results. The existence of a chemical difference between a habitual and a novel stimulus is difficult to reject. State-dependent learning in its turn may be based on the chemical specificity raised during learning.
During habituation, a neuron acquires the ability to regulate an excitability of its own membrane depending on the biological significance of stimulus novelty. In response to the habitual stimulus, the neuron displays lower excitability than in response to the novel stimulus. This regulation is based on chemical processes the nature of which is not yet known. All that can be suggested is that chemical processes arising in the neurons after the action of the stimulus changes after habituation and that the result of action of the novel stimuli differs qualitatively. Experiment allows making this conclusion without any a priori knowledge of the underlying anatomy, physiology or biochemistry. This conclusion does not depend on knowledge about neural pathways for the tactile and light stimuli, types of the neurotransmitters, etc. However, there is the question, whether the same synapses induce different chemical reactions before and after habituation, or new chemical reactions were induced by new synapses, which were activated, as the result of habituation. The second possibility looks less probable, since habituation, disinhibition and other forms of behaviorally-related plasticity may be induced by "artificial synapses": micro-electrodes for application of electrical current or drugs [1157, 662, 1419, 1173]. The chemical process evoked within a neuron by a stimulus is rebuilt after habituation. The problem of finer specificity is as yet open. It is much more difficult to hope that informational significance may provide the chemical difference between memory elements (for example, different habitual or different novel stimuli), since such a supposition is too complex to be accepted unconditionally. A wide variety of memory elements implies the participation of informational macromolecules in a quantity that exceeds the genetic memory. As a lesser assumption, chemical specificity may only provide a difference in the biological significance of the reactions. At a minimum, this assumption is, evidently, correct. There are also intermediate assumptions. Chemical specificity may correspond to classes of objects (faces, foods, places, dangers, etc.). Elements of memory may also correspond to specific chemical processes in the given brain, while the same elements differ in other brains. At present we may conclude that, at least during the fresh memory (tens of minutes), some aspects of information store have a chemical nature.