Halorhodopsin
In 1980, halorhodopsins (HRs) were for the first time described in the plasma membranes of Halobacterium salinarum[38- and in other haloarchaea such as Natrono-monas pharaonis. For these haloarchaeal extremophiles, chloride is a crucial growth parameter because the viability of haloarchaea depends on hypersaline environments like salt ponds or salinas. In the cytosol, the high osmolarity of the environment is counterbalanced by almost iso-osmolar KCl concentrations that can be as high as «4 M. During cell growth, the uptake of potassium chloride occurs actively by the combined action of potassium channels and halorhodopsins. The latter energize this process by acting as light-driven, inwardly directed chloride pumps.[39- Apparently, the light energy that is converted by HR contributes substantially to the maintenance of an osmotic balance during cell growth. Alternatively, passive salt import can be mediated in the dark by potassium and chloride channels.
Halorhodopsins exert slightly different affinities for the halides (Br">Cl", I") which are pumped against their electrochemical gradients.1-40-1 The halorhodopsins of H. salinarum and N. pharaonis can be produced by homologous and heterologous overexpression in large amounts.1-41,42-1 Therefore a plethora of biophysical techniques was applicable in the past to reveal details about the transport mechanism, the photocycle kinetics, and the primary photoreaction of the chromophore (reviewed in Refs. [21-, [40-, and [43-).
Halorhodopsins define one subfamily of the microbial rhodopsins which also comprise subfamilies like the light-driven, proton-pumping bacteriorhodopsins (BR) and the sensory rhodopsins with sequence identities to each other of 25-35%.[44- The structural similarity to other microbial rhodopsins, i.e., an architecture with seven transmembrane a-helices (numbered from A to G), was initially proven by cryoelectron microscopy on 2-D crystals of halorhodopsins1-45"47-1 which were spontaneously formed in vivo during the homologous over-expression in H. salinarum.
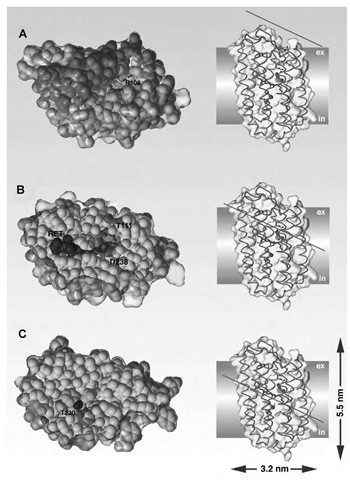
High-resolution data at 1.8 A were obtained by X-ray crystallographic analysis of H. salinarum halorhodopsin 3-D crystals1-48-1 which were grown in a lipidic cubic phase.[49- In these crystals, halorhodopsin (molecular weight: 27.0 kDa, 253 amino acids) assembles to trimers (Fig. 3A). The layer-like crystal packing highly resembles the organization of purple membranes which are formed in vivo by bacteriorhodopsin. The quaternary structure of the HR trimer differs significantly from the bacteriorhodopsin trimer because the monomers are tilted by 11° relative to the BR trimer and involve van der Waals packing between the C helices in addition to interactions between the B and D helices.[48-1 The halorhodopsin structure shows that a lipidic plug consisting of the fatty acid palmitate is encircled by the HR trimer (Fig. 3A). This surprising association of halorhodopsin with a fatty acid was shown before by biochemical analysis,[50- but its implication for the action of halorhodopsin is unclear. At least, a change in the photocycle kinetics and the pKa of the protonated Schiff base was found upon the removal of palmitate[51- whose carboxylate group is approximately 11 A away from the chloride that is bound to the transport site.[48] The most appealing feature of the ion- and signal-translocating microbial rhodopsins is the presence of an all-trans retinal chromophore that is bound as a protonated Schiff base to a conserved lysine residue, K242 in helix G (Figs. 3B and 4B). This chromophore causes the purplelike color of the unphotolyzed HR state with an absorbance maximum 1max of 578 nm. Illumination of this HR state causes the formation of a red-shifted intermediate, the K-state (1max~ 600 nm), by photoisomerization of the retinal from the all-trans to a 13-cis, 15-anti configuration and an accompanying flip of the N-H dipole of the protonated Schiff base. The 23 kcal/mol of remaining photon energy are mostly stored in the K-state by a twisted conformation of the retinal chromophore and drive the on-going conformational changes of the protein which accompany unidirectional ion transport. The flip of the Schiff base N-H dipole was indicated by a small, negative electrogenic charge shift upon K-state formation.[52]
Fig. 3 Halorhodopsin. (A) Trimeric arrangement of halorhodopsin as viewed from the extracellular side. The bound retinal (stick representation), chloride (green sphere), and palmitate groups (CPK model) are highlighted. Helix assignments are shown in blue. (B) Side view on halorhodopsin showing the translocation pathway of chloride. n-Helical distortions in the a-helix bundle are shown in blue. (C) Chemical environment of the chloride that is bound in the transport site. Hydrogens are shown as white spheres, chloride in green, and the nitrogens of the protonated Schiff base (PSB) and W112 in blue. Figure C was reprinted with permission of Nature.
Fig. 4 Steric restraints on the chloride translocation pathway through halorhodopsin. The location of the cross sections through halorhodopsin is indicated by a red line. The chloride ion in the transport site is highlighted as green sphere; the retinal chromophore is shown in blue and buried water molecules are shown in red. (A) View from the extracellular side. (B) The transport site for chloride. (C) The putative chloride release site. Surfaces of the internal cavities are marked in purple.
The structure of the HR state of halorhodopsin shows that next to the protonated Schiff base a chloride is bound at a distance of 3.8 A (Fig. 3C[48]). Consequently, pumping of chloride from this transport site to the cytosol is directly linked to conformational changes of the chromophore and its vicinity which are organized in a well-ordered series of spectroscopically distinguishable photointermediates.[21,40] The observed electrostatic interactions between the chloride and the protonated Schiff base and another essential residue nearby, R108, were concluded before from the halide dependence of FTIR[53] and UV/VIS spectra.[54,55]
Because of the long distance and the relative geometrical arrangement, a strong hydrogen bond between the chloride and the Schiff base nitrogen is not expected in the HR state. However, the stabilization of the chloride in this central transmembrane portion involves the additional coordination to a cluster of three water molecules in the transport site (Figs. 3C and 4B). As in the structure of the ClC channel, the partial hydration around the Cl~ anion is supplemented by an H bond to the hydroxyl of the conserved serine S115 and a cluster of aliphatic hydrogens which are derived from protein side chains (Fig. 3C). Here electrostatic calculations suggested that these aliphatic side chains might contribute enthalpically to halide binding because of the numerous interactions between the C-H dipoles and the chloride.[48] However, the affinities of chloride to the transport site of HR are not very high. Chizhov and Engelhard[56] estimated them from kinetic experiments to be in the millimolar range (N. pharaonis: «2.5 mM; H. salinarum: 10 mM).
Large conformational changes of the protein are required during the photocycle to open a transient pathway for chloride conduction because the retinal environment and the cytosolic half of HR are tightly packed (Fig. 4B and C). These changes might be transmitted by distortions in three of the a-helices (Fig. 3B): a P-helical segment in helix G (V239-F245), a P-helical segment at the cytosolic end of helix E (A178-W183), and a 3i0-like helical segment in helix C (L110-A113). After photoisomerization, the peptide planes of the first n-helical segment, which are adjacent to K242, reorient and change the H-bonding pattern of the transport site in the early photocycle intermediates. The second P-helical segment was suggested to be a hinge for the movements of helix F that accompany the formation of late photo-cycle intermediates.1-57-1
The only a-helical distortion that is not found in other microbial rhodopsins is the 3i0-like helical segment of helix C.[21] This stretch includes T111 which corresponds to D85 in bacteriorhodopsin, the primary proton acceptor. Compared to D85 of bacteriorhodopsin, the T111 residue is retracted by 1.8 A out of the chloride-occupied transport site of halorhodopsin. Recently, it was suggested that T111 might drive like a piston the chloride toward the cytosolic side of the chromophore by restoring the regular a-helical H-bonding pattern after photoisomerization.[21] Interestingly, the hydroxyl of T111 is not contributing to Cl~ binding as shown by the crystal structure of HR and transport studies of the T111V mutant.[58]
While K-state formation occurs within picoseconds and is too fast to cause a major displacement of the chloride from the transport site, the subsequently, in about 1 psec, reversibly formed L-state (1max ~ 520 nm) coincides with the movement of the halide to a second chloride binding site in the cytoplasmic half of HR.[21,48] The putative, second chloride binding site is empty in the HR state (Fig. 4C), but borders directly to the Schiff base and is completely occluded from cytosolic access.[48] Consequently, after light-triggered movement of a chloride into this cytoplasmic release site, substantial conforma-tional changes are required in the cytoplasmic half to establish free exchange with the cytosolic milieu. In N. pharaonis halorhodopsin, the transport site lowers its chloride affinity from 2.5 mM to 1.1 M after photoex-citation,[56] while the affinity of the cytoplasmic release site is almost unchanged («5.7 M.[59]).
Interestingly, the transport specificity Br~>Cl~ arises from the better binding of Br~ to the cytoplasmic release site, but not to the transport site.[54] Alternative or in addition to the piston model, where movements of the T111 residue drive the ion translocation, some form of ”ion-dragging” was considered before where the halide ion follows the flipped dipole of the N-H Schiff base bond and keeps a strong hydrogen bond with the protonated Schiff base.[48] A subsequent opening of an extracellular pathway from the cytoplasmic release site toward the protein surface was attributed either to a transition between the L2 and O states[60] or to a spectroscopically silent transition between two L-like intermediates.1-56,61-1 The chloride transport along the extracellular pathway occurs by passive diffusion during the later part of the photocycle.[62] However, the precise order of the further photocycle intermediates is still unclear and might be, for halorhodopsin, even a function of the halide concentration and the source organism. Chloride uptake occurs presumably along an access channel whose outer rim on the extracellular side is lined by aliphatic side chains (Fig. 4A) which may support the deformation of the hydration shell of the incoming halide ion.
Converting Proton Pumps to Chloride Pumps
A single mutation in bacteriorhodopsin, aspartate 85 to serine or threonine, suffices to convert the specificity of bacteriorhodopsin from proton to chloride transport.1-63-1 The photocycles of halorhodopsin and these mutants resemble each other, but the photocycle of the D85T mutant additionally indicates that chloride uptake becomes a rate-limiting step compared to halorhodopsin.[64,65- This might be caused by a sterically hindered access of chloride from the extracellular side to the transport site or by the lack of a hydrophobic ridge around the extracellular entrance site as in HR. Besides the 10-fold reduced transport efficiency, the D85T mutant also exhibits a 20-fold relaxed affinity to Cl",[64,65- for which the structural reasons are not delineated. Furthermore, in the D85T mutant, the Cl" translocation is accompanied by a deprotonation of E204, which belongs to the extracellular proton release group of bacteriorhodopsin but is missing in halorhodopsin, as this residue is there replaced by a neutral threonine.[21-
Apart from T203 in halorhodopsin which is predicted to line the cytosolic release site for Cl" (Fig. 4C), no other residues essential for halide transport were identified by mutagenesis in the cytoplasmic half of halorhodopsin[58-or the bacteriorhodopsin D85T mutant.[65- Consequently, the walling of the postulated cytosolic exit pathway for Cl" can be rather unspecific to promote the passive diffusion of Cl" into the cytosol.[62-
Halorhodopsin is converted to an outwardly directed proton pump, if the chloride in the transport site was replaced by an N3" anion.[66- As a result of a pKa of 4.75 in solution, this azide can act as a proton-accepting group such as D85 in bacteriorhodopsin. The possibility to convert the transport specificities of both bacteriorhodop-sin and halorhodopsin from proton to chloride transport and vice versa implies that in these proteins, proton and halide transports depend on similar mechanisms. During the L! M transition of bacteriorhodopsin, a proton is transferred from the protonated Schiff base to the deprotonated D85. Among the models that have been suggested to rationalize the unidirectionality of this step (summarized in Ref. [67-), the so-called hydroxide hypothesis can reconcile these observations. This hypothesis states that a water molecule next to the Schiff base becomes firstly deprotonated by D85 after photoisome-rization of the chromophore. The hydroxide then moves toward the cytosolic side of the chromophore like Cl" in halorhodopsin.[67- There, it abstracts a proton from the protonated Schiff base so that as a net result, a water molecule is pumped inwardly and a proton outwardly. The hydroxide hypothesis suggests that the ion-translocating archaeal rhodopsins generally catalyze the passage of anionic species across the photoisomerized retinal where only steric and electrostatic properties of the transport site determine the OH" vs. Cl" specificity.
CONCLUSION
The structural studies on natural, halide-conducting membrane proteins show that halides are stabilized in the central region of lipid bilayers by similar mechanisms as postulated before for the transfer of halides from aqueous to apolar phases, i.e., ''fingering'' of the aqueous solvent into the transmembrane region and electrostatic compensation of the halide's charge. The charge compensation involves arginine side chains such as in halorhodopsin or the Omp32 porin or at least the alignment of the dipole moments of several helices toward the bound anion such as in the ClC channels. Apart from this, the chloride binding in these proteins occurs without a highly ordered coordination shell as observed before in cation channels. For the potassium channels, it was noted that K+ ions are highly regularly coordinated in the selectivity filter in two alternative coordination geometries:1-37-1 a square-antiprismatic coordination sphere with eight oxygen atoms and an octahedral geometry with six oxygen ligands which are derived either from water or from carbonyl groups of the selectivity filter. These coordination geometries coincided with structural data from potassium-selective antibiotics such as nonactin or valinomycin and confirmed that there are common principles for the stabilization and the conductance of cations in lipid bilayers by K+-selective ionophores and channels.
A common feature of the structures discussed above is certainly the partial lining of the selectivity filter by hydrophobic residues. According to the theory of large-ion conductance, such an apolar channel lining might impose an energetic penalty for water intrusion into the selectivity gate and induce the observed anion specificity by excluded volume effects.1-9-1 Unfortunately, this theory might be hard to prove by mutagenesis and biophysical analysis of natural membrane proteins because small differences in the geometry of the channel could exert large differences for the energetics of water intrusion. What is needed is hence a much simpler and, in steric terms, more stringently defined system than the ClC-like chloride channels or the porins.
Biochemical and biophysical data about small, natural transporters such as cryptdin-3[18] or synthetic model compounds capable of transmembrane halide transport[68] have been reported since only a few years (Fig. 5). The design of most of the synthetic halide transporters is based on peptidic backbones. In the case of a specificity-modified, dimeric alamethicin derivative, a mild anion preference was built in by introducing a lysine residue close to the C-terminal end.[69] However, the observed anion preference was caused by a drop of the cation conductance, while anion permeability was unaffected in comparison to the parental alamethicin. Another more promising strategy followed by Schlesinger et al.[70] was inspired by the conserved motif GKxGPxxH that is present in the anion pathway of ClC chloride channels. By attaching the heptapeptide GGGPGGG to a lipidic anchor (Fig. 5), these authors obtained chloride conductivities with >10 CP/K+ selectivity. Furthermore, they could show that these synthetic chloride membrane transporters (SCMTRs) exhibit voltage gating similar to natural chloride channels. Their conductance crucially depends on the presence of the proline kink[71] and the precise length of the lipidic moiety.[72] Interestingly, this design lacks a direct electrostatic compensation of the chloride charge. The distortion in the lipid bilayer by the proline kink might hence indicate a mechanism which relies on the ”fingering” of partially dehydrated chlorides into the apolar region.
Another evidence for the relaxed structural requirements for transmembrane halide conductance is the observation that peptides which are derived from the transmembrane regions of CFTR or the GABA receptor readily form chloride channels in vitro.[73,74] As these peptides might have a pharmaceutical potential in the treatment of CFTR deficiency, i.e., cystic fibrosis, they are momentarily subject to further optimization in terms of solubility and conductance properties.[75] While these approaches to halide channels used peptidic backbones for the transmembrane region, one of the first successful designs of a nonpeptidic chloride transporter utilized a calix[4]arene as chemical scaffold (Ref. [76], Fig. 5).
Certainly, further high-resolution structural studies on synthetic and natural halide transmembrane transporters will be needed before our view about the transport of halides is similarly concise as it is nowadays for the transport of cations.
Fig. 5 Synthetic halide transporters. I=SCMTRs,[70] II=alamethicin derivative alm-K16,[69] IIIa=C-K4-M2GlyR,[75] IIIb=N-Kr M2GlyR,[78] IV=calix[4]arene tetrabutylamide.

![Synthetic halide transporters. I=SCMTRs,[70] II=alamethicin derivative alm-K16,[69] IIIa=C-K4-M2GlyR,[75] IIIb=N-Kr M2GlyR,[78] IV=calix[4]arene tetrabutylamide. Synthetic halide transporters. I=SCMTRs,[70] II=alamethicin derivative alm-K16,[69] IIIa=C-K4-M2GlyR,[75] IIIb=N-Kr M2GlyR,[78] IV=calix[4]arene tetrabutylamide.](http://what-when-how.com/wp-content/uploads/2011/03/tmp70E158_thumb1_thumb.jpg)
