Nanoparticle Formation in Dendrimers
The most commonly used and studied dendrimers (commercially available) are poly(amidoamines) (PAMAM), whose structure is presented in Ref. [24]. By varying the dendrimer size (dendrimer generation) and metal compound loading, one can vary the size of nanoparticles.
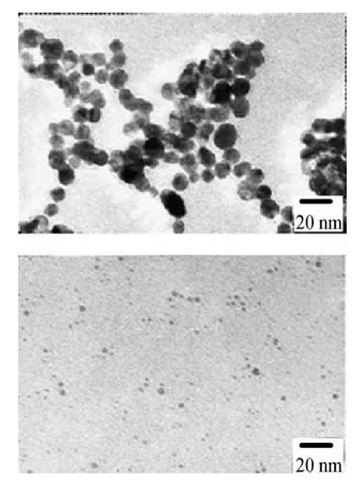
Dendrimers were used for stabilization of gold and silver nanoparticles with subsequent self-assembling of metal-containing dendrimers on the surface with monolayer formation.[25] Poly(amidoamine) dendrimers with terminal hydroxyl groups were used as templates and stabilizers for controlled synthesis of monodisperse, catalytically active nanoparticles.[26] In the first step, metal ions were absorbed by the dendrimer as a result of the formation of metal complexes with amino groups. Subsequent reduction results in the formation of metal nanoparticles encapsulated in the dendrimers. For hydroxyl-terminated dendrimers, a deficiency of metal exchange between dendrimer molecules provides very narrow particle size distribution and subtle control over nanoparticle growth: The nanoparticle size is exactly determined by the amount of metal atoms loaded in the dendrimer. These metal-containing dendrimers are water-soluble and very stable (no precipitation is observed for months). It is considered that a metal particle is formed in the cavity of the dendrimer. This location provides a certain stabilization of the particles. At the same time, the presence of functional (amino) groups allows additional stabilization of nano-particles. Metal particle formation resembles the growth of nanoparticles in the cross-linked cores of block copolymer micelles when exchange between micelles does not occur. If PAMAM contains surface (terminal) amino groups, exchange between dendrimer molecules results in a broader particle size distribution than for PAMAM with terminal hydroxyl groups (Fig. 8).[27]
Fig. 8 Electron micrographs of gold colloids and histograms of particle size distribution at molar ratio of surface amino group of G5 and HAuCl4: 1:1 (top) and 4:1 (bottom).
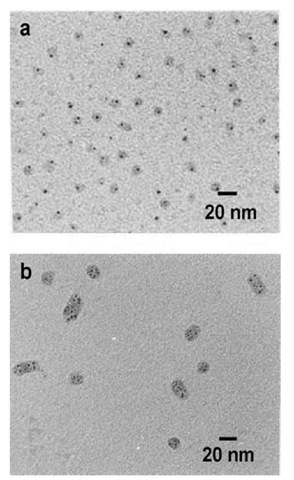
Fig. 9 Transmission electron micrograph of gold containing G8 PAMAM (a) and G10 PAMAM (b) dendrimers obtained for 1:1 loading and slow reduction. In both cases, the dendrimers have been stained with phosphotungstic acid.
Dendrimer generation is an important factor to influence the particle characteristics.1-28-1 Gold colloid formation upon reduction of a gold salt precursor in protonated PAMAM was studied to follow the influence of reaction conditions and dendrimer generation on the resulting polymer nanocomposite colloids.[29] Methods such as TEM, SANS, and SAXS show that the gold particles are formed inside the dendrimer and located offset from the center (probably in a dendrimer cavity). Lower generation dendrimers aggregate when nanoparticles are formed. Dendrimers of generation 6-9 can template one gold nanoparticle per dendrimer molecule; the number of gold atoms added per dendrimer determines the particle size. These data well agree with those described in Ref. [26]. For generation 10, multiple smaller gold particles per den-drimer were observed and dendrimers aggregated (Fig. 9). Poly(propyleneimine) dendrimers with stearyl end groups, combining both hydrophilic and hydrophobic moieties, were also used for metal particle formation.[30] These dendrimers form inverse micelles in toluene. While the initial dendrimers have a spherical structure with a collapsed core, solubilization of metal salt hydrate leads to the formation of cylindrical multidendrimer structures with swollen, metal-salt-filled dendrimer cores. When the gold salt inside the dendrimers is reduced to form colloidal particles, the cylindrical structure breaks up and spherical nanoparticles are formed. In so doing, the particle sizes are larger than would be expected if the gold-salt loading of one dendrimer formed one particle, indicating that the ions from several dendrimers are combined. Apparently, dendrimers of this kind provide no control over nanoparticle growth. In addition, these den-drimer aggregates, although resembling block copolymer micelles, are less defined and more complex. So advantages of such amphiphilic dendrimers are not evident.
Along with metal particles, a number of semiconductor particles, CdS,[31-33] CdSe,[34] and complex core-shell CdSe/ZnS,[32] were successfully prepared in dendrimers. The absorption and emission of the CdS/PAMAM (hydroxylated) systems are a function of the generation of the dendrimer that is related to the dependence of nanoparticle size on dendrimer generation and the dependence of optical properties on a semiconductor nanoparticle size.[32]
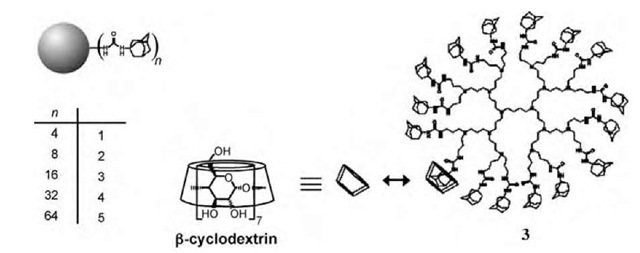
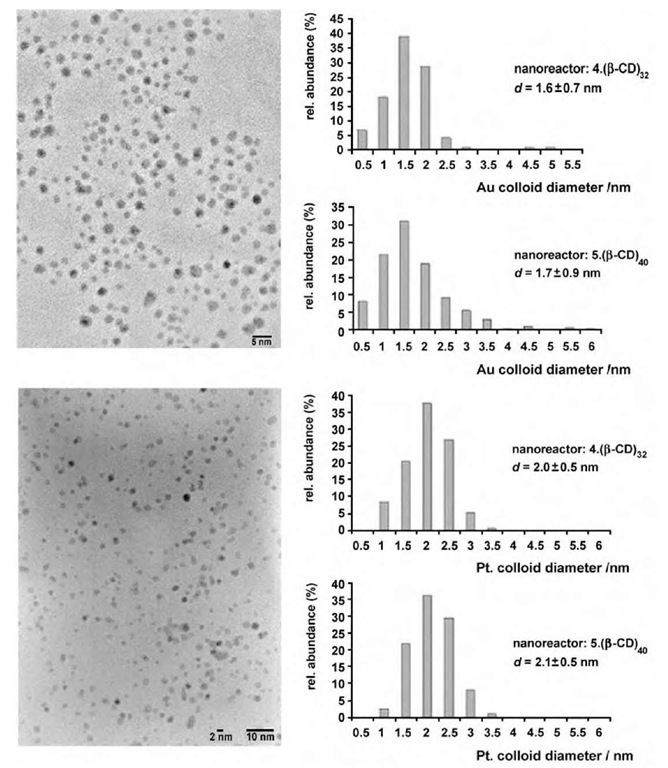
Aqueous assemblies of adamantyl-derivatized poly-(propylene imine) (PPI) dendrimers and p-cyclodextrin (p-CD) (Fig. 10) have been used as nanoreactors in the preparation of gold and platinum nanoparticles in wa-ter.[35] These particles have been formed by the reduction of aurate or platinate anions in the presence of the generation 4 (4_(p-CD)32) and 5 (5_(p-CD)40) assemblies (Fig. 11). Lower generation assemblies did not provide stable nanoparticles. The authors believe that the persistent shape of the adamantyl-derivatized dendrimers and the dense shell of adamantyl-p-CD complexes provide a kinetic barrier for nanoparticle escape, thus prolonging their lifetime. However, particle size distribution inside p-CD-modified dendrimers is not particularly narrow,[35] thus demonstrating poorer control over particle size than in hydroxy-terminated PAMAM.
Fig. 10 Poly(propylene imine) dendrimer generations 1-5, solubilized by P-cyclodextrin.
Nanoparticles in Polyelectrolyte Microgels
Microgels are gel spherical particles having diameters in a nanometer range.[36,37] Internal cross-linking leads to stability of their sizes and properties, while their size (in nanometer range) ensures formation of colloidal solutions. When these microgels are formed by polyelectrolytes, they contain charged groups allowing ion exchange and solubilization in water. This allows considering microgels as nanoreactors for controlling nanoparticle growth. Gold nanoparticle formation was studied in microgels based on sulfonated PS.[38] Morphologies of nanoparticles formed are strongly determined by the degree of microgel cross-linking. The higher the cross-linking density, the higher the probability of the formation of spherical particles embedded in the microgels. The other key factor is the type of reducing agent. Fast reduction (NaBH4) in water leads to gold nanoparticles of 4.5 nm in diameter located in microgels. If NaBH4 is added in alkaline solution (0.1 N NaOH), it slows the reduction and results in 7-nm nanoparticles forming long ”threads.” Yet only 20% of microgels contain nanoparticles. Thus slow nucleation allows Au clusters and ions to migrate out of microgel areas where aggregation easily occurs. High-resolution TEM allows determining that particles grow from one nucleus and the microgel environment governs the particle shape. In a similar fashion, Pd and Pt nanoparticles have been grown in microgels. These metal-particle-containing microgels were also used as cotemplates (along with amphiphilic PS-b-PEO block copolymers) for meso-porous silica formation.[39] Here microgels play a dual role: They are nanoreactors for metal particle formation and pore-forming templates when mesoporous materials are formed.
Fig. 11 Transmission electron microscopic images of gold (top) and platinum (bottom) nanoparticles stabilized by the 5- (P-CD)40 assembly and size distributions of colloids stabilized by 4- (P-CD)32 and 5- (P-CD)40 (amine/metal=2:1, H2O, T=25°C).
Nanoparticle Formation in Functionalized Polysilsesquioxane Colloids
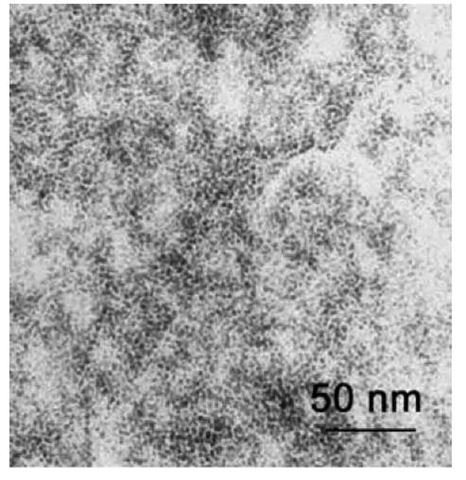
Synthesis of functionalized polysilsesquioxane based on hydrolytic condensation of functionalized silanes was recently described in Ref. [40]. Using N-(6-aminohex- yl)aminopropyltrimethoxysilane (AHAPS) as a precursor, well-defined colloidal particles composed of a nearly fully condensed poly(aminohexyl)(aminopropyl)silsesquioxane (PAHAPS) were synthesized. According to solid state 29Si CP-MAS (cross-polarization magic angle spinning) nuclear magnetic resonance (NMR), PAHAPS structure contains mainly C-SiO3/2 species. The sizes of PAHAPS colloids vary in the range 10-200 nm and depend on the reaction conditions: pH, solvent, and AHAPS concentration (Fig. 12). When hydrolytic condensation is carried out in water with no HCl added, self-assembling of AHAPS tails results in the formation of lamellar ordering with Bragg spacing of about 3.0 nm, which matches to two layers of fully extended AHAPS tails. When PAHAPS is fully or partially protonated (HCl is added to water), no ordering occurs. Similarly disordered structures are formed in THF, which is a good solvent for AHAPS tails. Unlike water, THF also facilitates cross-linking between colloids, so the colloids are attached to each other. Interaction of PAHAPS colloids with Pt and Pd salts followed by chemical reduction results in the formation of discrete metal nanoparticles stabilized within the colloids even at metal content of 30-45 wt.% (Fig. 13). The particle size depends on the type of metal compound and, in some cases, on type of the reducing agent. Particles formed after reduction of K2PtCl4 within PAHAPS measure ca. 1-2 nm in diameter when the fast reducing agent (NaBH4) is reduced. With the sluggish reducing agent (hydrazine-hydrate), both small particles of 1.5 nm and larger particles with diameters of 2.5-4.0 nm are formed. When metal precursor is K2PtCl6, very narrowly distributed particles measuring about 1.5 nm are formed for both types of reducing agents. Apparently, particle nucleation does not influence the particle size when mass transfer of the PtCl62 _ ions is restricted. Poly(aminohex-yl)(aminopropyl)silsesquioxane colloids form very strong elastic films because of the intercolloid interactions that makes possible the formation of freestanding films and coatings of different thicknesses on various supports.
Fig. 12 Transmission electron microscopic image of PAHAPS colloids obtained in water at a precursor concentration of 17 wt.%.
Fig. 13 Transmission electron microscopic image of Pt nanoparticles formed in protonated PAHAPS prepared in water at a concentration of 1.5 wt.%.
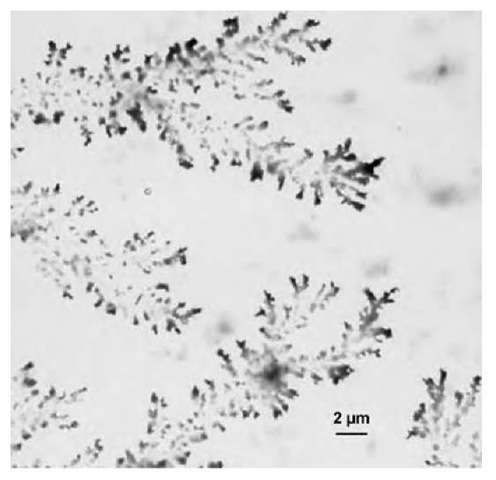
Another interesting feature of these particles is that dendrites differing in size and shape are observed to form from aqueous solutions containing PAHAPS colloids loaded with metal salts or metal nanoparticles (Fig. 14).[40] While formation of dendrites is common for a number of salts or metals,[41,42] it has not been reported so far for colloidal particles or nanoparticle-loaded colloidal systems. Because the size and shape of the den-drites produced here can be easily controlled, metal-loaded PAHAPS may be suggested as catalytically active membranes in which select surface coverage is important. Another possible application for PAHAPS loaded with metal nanoparticles is as a conductive layer between two surfaces, as described elsewhere[43] for pure Pd dendrite crystals. At the same time, unlike pure metals, metal-loaded PAHAPS possesses a number of practical advantages, including lower cost and greater structural stability.
Another precursor of interest for synthesis of polymer colloids using sol-gel reaction is octadecyldimethyl(3-trimethoxysilylpropyl)ammonium chloride (ODMACl). The hydrolytic condensation of this precursor both in acidic and basic solutions results in the formation of colloidal particles showing lamellar ordering with Bragg spacing d of about 3.6 nm. This Bragg spacing is significantly smaller than two layers of extended ODMACl tails, so one can assume either tilting of ODMACl chains (they are not perpendicular to the lamellar surface) or interpenetration of the tails of the two layers (or both). As the PODMACl colloids are much larger than twice the d spacing, they should have a multilamellar structure. Because PODMACl colloids contain ionic groups in their tails, they can be subjected to ion exchange that results in replacement of Cl_ ions for ions of interest: PdCl42 PtCl62AuCl4~. The subsequent reduction leads to metal nanoparticle formation within PODMACl colloids. Moreover, independently of reducing agent type, lamellar ordering is preserved, while particle size and shape strongly depend on the nucleation rate. With NaBH4, narrowly distributed spherical particles with a mean diameter of ~ 2 nm are obtained. When nucleation is slow, particle growth is directed by the lamellar ordering, so rod-like particles of 2x 13 nm are formed and their positioning is well regulated by the ordered structure. This suggests new opportunities for growing rod-like particles derived from different metal or semiconductors within ordered polymeric colloids. Such materials can be promising for tailoring optical, magnetic, and electrical properties of nanocomposites by tuning the particle shape.
Fig. 14 Transmission electron microscopic image of dendrites formed by PAHAPS colloids filled with Pd nanoparticles.
NANOPARTICLES FORMED ON THE POLYMER COLLOID SURFACE
Micrometer and submicrometer Au-shell PS latex beads have been prepared by combining the self-assembly and seeding methods.[44] The PS beads are first covered with positively charged poly(ethyleneimine) (PEI) via electrostatic interaction and hydrogen bonding; then, the Au shell is formed via the reaction of PEI-PS with NH2OH and HAuCl4. This method allows efficient control of the gold coverage and leads to relatively stable products. Using TEM, formation of Au clusters and gold nanoparticles on the surface of the PS beads was confirmed. The results suggest that the PEI polymer chains are probably stretched out on the surface of the PS beads. The UV-visible extinction spectrum for gold-covered particles is significantly affected by the plasmon resonance absorption.
For preparation of Au-shell magnetic particles, sulfo-nated polystyrene beads were first exchanged with Fe2+ ions under N2 gas flow and then treated with NaOH solution. The last stage involves heating the beads at 100 °C for 1 h. Iron oxide layer was later covered with a gold shell as described for Au shell/PS colloids. Self-assembled structures of Au-covered particles were induced by an external magnetic field.[44] This conductive grid was suggested for use as a tunable polarizer for microwaves and milliwaves, as the conducting lines that can be switched into a nonconductive state by an external field. Waveguides for plasmon, i.e., excitation of the plasmon resonance at one end of the lines and detection at the other end, is another interesting application.
Well-dispersed Ag nanoparticles were formed in situ on the surface of poly(N-isopropylacrylamide, PNI-PAAm)-coated polystyrene microspheres.[45] The surface-grafted PNIPAAm chains serve both as steric stabilizers to prevent the flocculation of the polystyrene particles and interact with silver salts with subsequent adsorption of the forming Ag nanoparticles onto the surfaces of the microspheres. By varying the concentrations of initiator and silver nitrate, the particle sizes and distributions for both Ag nanoparticles and polystyrene microspheres can be altered. As PNIPAAm is a well-known, temperature-sensitive polymer, the PNIPAAm-protected Pt colloids show unusual temperature dependence of activity in the aqueous hydrogenation of allyl alcohol, which can be explained by different density of PNIPAAm layer at different temperatures. In addition, Pt colloids on the microspheres show higher activity than the commercial Pt/C catalyst and retain high activity on recycling in the same reaction. Polystyrene microspheres with silver nanoparticles can be used as the active substrates for surface-enhanced Raman scattering, chemical, electronic, and optical sensors, and photocatalysts for solar energy conversion.
Another example of PS beads covered with nanopar-ticles is described in Ref. [46]. Surface-functionalized PS microbeads and nanobeads were prepared by grafting the p-acetoxystyrene monomer during the last 30 min of the fabrication of polystyrene bead core by emulsifier-free emulsion polymerization followed by hydrolysis of the acetoxy group by a base. The size of the resulting beads is mostly dictated by the size of the core. Hydroxyl-derivatized polystyrene microspheres have been used for anchoring catalytically active silver and ruthenium nanoparticles. This was performed by adsorption of preformed nanoparticles on the functionalized bead surface. The bead formation, surface functionalization, and coating with metal nanoparticles were studied using scanning electron microscopy (SEM), TEM, energy dispersive X-ray spectrometry (EDS), Fourier-transform IR spectrom-etry, and Auger analysis.
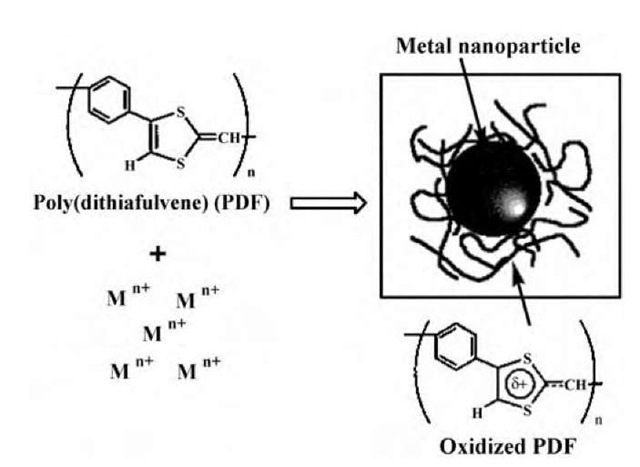
Fig. 15 Schematic illustration of the formation of the PDF-protected metal nanoparticles via reduction of metal ions by the p-conjugated, electron-donating PDF.
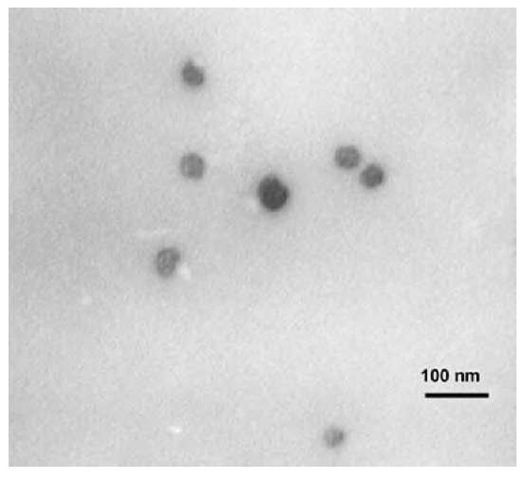
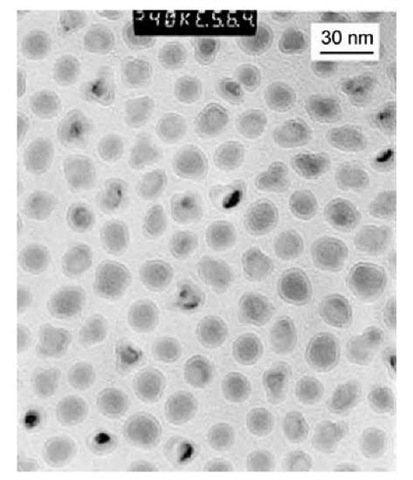
Fig. 16 Transmission electron microscopic image of PIB-coated nanoparticles made with 80 g of Fe(CO)5 and 11 g of PIB-TEPA (tetraethylenepentamine).
NANOPARTICLES WITH ADSORBED POLYMER LAYER
Any nanoparticle synthesis in polymer solution normally results in the adsorption of polymer molecules on the nanoparticle surface. Usually, these materials are not considered as metalled polymer colloids. Nevertheless, in some cases including ones cited below, adsorption or chemical interaction of polymers with nanoparticles is accompanied with formation of a polymer layer of different nature than that used for nanoparticle synthesis.
Reduction of Pd, Pt, or Au ions in dimethyl sulfoxide (DMSO) solution by a p-conjugated poly(dithiafulvene) (PDF) having strong electron-donating properties resulted in the adsorption of oxidized polymer on the forming nanoparticle surface, which protected the metal nanopar-ticles (Fig. 15).[47] As found, all the DF units of PDF were uniformly oxidized by electron transfer, which means that they participated in the reduction, and resided on the particle surface. The average size of the formed Pt nanoparticles increased with the increases of the PDF concentration and reaction temperature. All of the Pt nanoparticles formed at different temperatures showed narrow size distribution, high dispersibility, and great thermal stability.
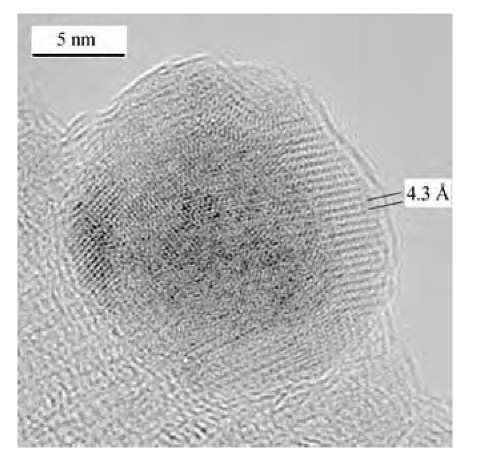
Magnetic materials comprised of polymer-iron nano-particle composites have been prepared by thermal decomposition of iron pentacarbonyl in the presence of ammonia and several types of polymeric dispersants.[48] The nanoparticles consist of metallic cores, each of which is coated with a strongly bound polymer layer (Fig. 16). The polyisobutylene- and polyethylene-based dispersants lead to more uniform particle sizes and the materials are composed principally of individual core-shell particles. Large, complex particles, formed by the aggregation of smaller particles, as well as simple core-shell particles, were obtained with polystyrene-based dispersants in 1-methylnaphthalene. Aggregation leading to complex particles was found to be a result of a mismatch between the solubility parameters of the dispersant and the reaction solvent. When the solvent was better matched with the solubility parameter of polystyrene, simple core-shell nanoparticles of fairly uniform size were obtained. Electron diffraction revealed that the core material is iron. High-resolution TEM showed highly ordered or crystalline regions within the polymer shell, possibly because of the dense packing of the strongly bound dispersant chains (Fig. 17). Magnetic interactions between particles cause formation of secondary structures such as clusters, coils, loops, and strings of particles. The nanocomposites can be dispersed in organic solvents or cast as films. The polystyrene-iron composites are rigid solids at room temperature but can be melted at temperatures of 65-100°C. The nanoparticle composites showed different magnetic behavior depending on the particle size. Samples with the smallest particles proved to be superparamagnetic but their saturation magnetizations were low. Hysteresis was observed for materials with larger particles, and the materials possessed larger magnetizations. The magnetization was correlated with the particle size where samples with larger particles showed higher magnetizations. The samples with higher iron contents, achieved using higher iron pentacarbonyl loadings or by removing unbound polymer dispersant, showed the highest magnetization.
Fig. 17 High-resolution TEM image of PIB-coated nano-particles made with 60 g of Fe(CO)5 and 11 g of PIB-TEPA. Lattice planes are visible in the shell region of the particles.
CONCLUSION
This entry clearly shows several approaches to constructing nanocomposite polymer colloids containing nanopar-ticles. In all cases, polymer colloids are soluble in organic or aqueous media (depending on the exterior of the colloids) and retain solubility after nanoparticle formation. This key feature allows the formation of thin deposited or freestanding films (the latter were obtained with block copolymer micelles and functionalized polysilses-quioxane colloids) that makes possible a number of important applications for nanolithography in optical and magnetic materials. For catalytic applications, both homogeneous (solutions) and heterogeneous systems (after deposition on the support) proved to be promising with the polymer colloids filled with nanoparticles. It is worth mentioning that the polymer nanoenvironment significantly changes the nanoparticle properties via modification of the nanoparticle surface with polymer groups; therefore the choice of polymer type and structural organization of polymer colloids play a crucial role in material properties and should be taken into consideration for specific material application.