INTRODUCTION
Carbon is everywhere around us. Its stable crystalline phase is graphite, but under some circumstances, it can be converted into its most interesting phase for applications—diamonds. Naturally, a diamond is produced inside the Earth’s mantle by a high-pressure-high-temperature phase transformation of graphite. It is metastable and that is the reason why it can be recovered in mines after having migrated toward the most external shells of the Earth.
Its unsurpassed hardness, excellent transport properties, transparency, and inertness make a diamond a material of choice for many industrial applications. Synthetic diamond, produced by a high-pressure-high-temperature treatment of graphite, or by ion bombardment, is now commonly used in industries. But what happens to the carbon phase diagram when the sample size reaches the order of several nanometers?
OVERVIEW
After the discovery of diamond inclusions (several nanometers in size) in some meteorites that had fallen on Earth, some groups have theoretically studied the relative stability of graphite and diamond as a function of particle size.
The first attempt to understand the stability of diamonds at the nanoscale was published by Badziag et al.[1] They computed the binding energy of diamondlike and graphite-like carbon clusters using fixed energy values for carbon-carbon and carbon-hydrogen bonds. They found that below a size of 3-6 nm and a number of 100-21000 carbon atoms, diamond clusters become more stable than their graphitic counterparts. After that precursor study, several groups tried to model the relative diamond-to-graphite stability with more sophisticated models. A charged cluster model was presented by Hwang et al.[2] and Jang and Hwang.[3] At that time, the crossover between diamond and graphite was predicted to occur for clusters containing 400 atoms. In comparison with Ref. [1], the model used there was purely electrostatic. Other charge lattice calculations by Gamarnik[4] predict the reversal of stability to occur between 4.3 and 10.2 nm, depending on the temperature. It is worth noting that although these models have totally different ingredients and neglect the detailed structure of the clusters, such as specific surface structure or different degrees of hydrogen surface passivation, they reach the same conclusion that a diamond becomes more stable than graphite below 3-10 nm. Indeed, diamonds of several nanometers in diameter have been found or produced in a large variety of environments, as we will discuss in ”Nanodiamond Sources.” Then, we will present an overview of the properties of nanodiamonds, both as isolated particles and as assemblies in films, and we will show how promising these carbon nanoparticles are for tomorrow’s applications.
NANODIAMOND SOURCES
Nanodiamonds in the Sky
Nanoscale diamonds were discovered in 1987 by Lewis et al.[5] in meteorites. Not all meteorites contain diamonds. At this time, nanodiamonds have been found in specific types of meteorites, the so-called ”carbonaceous chondrites.” Two major sources of nanodiamonds are the ”Allende” chondrite (C3V type), which weighed several tons when it fell on Mexico in 1969, and also other types of chondrites, such as the Murchison meteorite (type C2).
The nanodiamonds found in these meteorites have a lognormal size distribution, with a median diameter of 26 A, corresponding to roughly 1060 atoms. The shape of the distribution has been interpreted to be caused by growth followed by partial conversion of small grains to larger ones. The particles were shown to contain impurities, mainly hydrogen, nitrogen, and oxygen, principally in -COOH groups.[6] It is worth noting that in some cases, such as C2-type chondrites, a diamond (in its nanoscale form) is up to five times more abundant than graphite. Further analysis of meteoritic nanodiamonds by Amarti et al.[7] revealed the nanodiamond content of the C2 meteorite to be ~ 400 ppm and attributed the shape of the size distribution to a condensation process.
The major question arising from those discoveries is: How and when were those diamonds produced? Daulton et al.[8] performed a detailed high-resolution transmission electron microscopy (HRTEM) study of a large quantity of nanodiamonds coming from meteorites, as well as those produced by means of detonation or chemical vapor deposition (CVD). Their work concludes that meteoritic diamond features (morphology, type of eventual twinning) are much closer to the low-hydrogen-pressure CVD process than to the high-temperature-high-pressure detonation.
These findings are of much interest to the astrophysicists community because chondrites are primitive meteorites that formed before the solar system. Their structure and content provide information on nuclear and chemical processes in stars and in the interstellar medium. There still remained some doubts about the fact that the nanodiamonds found in the meteorites are actually presolar.[9] Dai et al.[10] have studied other nanodiamonds, with structural features similar to the meteoritic samples, which have been found in interplanetary dust particles originating from comets or asteroids. They found that there are very few nanodiamonds in those particles— infinitely fewer than in chondrites. Because comets are objects that formed earlier in the solar system than meteorites, this seems to indicate that the nanodiamonds found in the meteorites are not presolar at all.
This hypothesis is corroborated by the discovery of unexpected lines in the infrared spectra of 12 warm supergiants,[11,12] which are also carbon-rich protoplane-tary nebulae. A broad absorption line centered at 21 mm wavelength was attributed to large polycyclic aromatic hydrocarbon (PAH) molecules or some partially hydro-genated fullerenes,[13] before being attributed to nanodiamonds by Hill et al.[14] In that study, the observed infrared absorption from the interstellar dust is shown to be comparable to either nitrogen-rich nanodiamonds, or to nanodiamonds containing vacancies and/or interstitial atoms. They also noticed that some absorption features could be explained by the relaxation of part of the nanodiamonds surface toward sp2 carbon configuration. By fitting the absorption spectra with those of terrestrial diamonds, they estimate the size of these interstellar nanodiamonds to be around 2.6 and 3 nm, exactly as for meteoritic diamonds.
Other hydrogenated nanodiamond signatures have been indirectly evidenced by Van Kerkhoven et al.[15] around some other types of stars. In those objects, the nanodia-monds are thought to be hydrogen-terminated, with some surface reconstructions, and could have a size ranging between 1 and 10 nm. The main conclusion of that study is that the nanodiamonds are produced in situ in the disks of stellar objects. Thus they should be present everywhere. The authors suggest that nanodiamonds are indeed present everywhere in space, but remain undetected because of the dehydrogenation of their surface for reconstruction, with the surface carbon getting sp2-bonded.
Nanodiamonds in Detonation Soots
As one can see, nature seems to favor the appearance of diamond nanoparticles. But it is not only in the cosmos that nanodiamonds can be found.
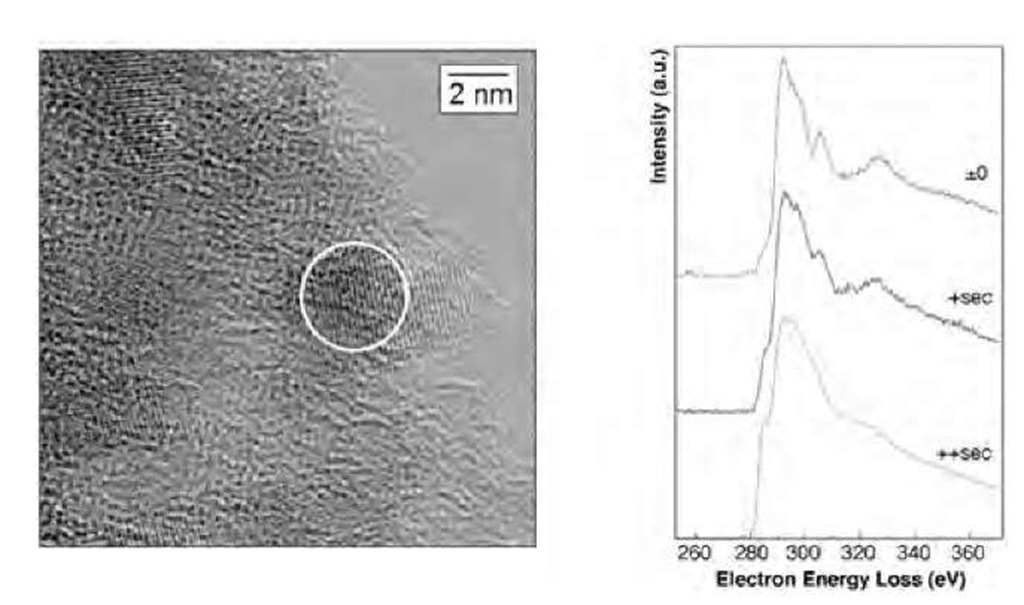
Indeed, pure trinitrotoluene (TNT) detonation has been shown to produce, among other carbon structures, nano-crystalline (NC) diamonds with diameters of about 10 nm.[16] By mixing TNT with some other solids such are RDX (cyclotrimethylene trinitramine—C3H6N6O6), TATB, or NIGU, and by detonating the mixture in an inert gas atmosphere, most spheroidal diamond particles produced were 4 nm in diameter.[17] Because of those first studies, the synthesis of nanodiamonds by detonation has been optimized and detonation-produced diamonds are now even commercially available. These nanodiamonds are often called ”ultra dispersed diamond” (UDD) because of their very narrow size distribution. A thermo-dynamic model has been proposed by Viecelli et al.[18] in which the nanodiamond formation is produced from nanometric liquid droplets. Aleksenski et al.[19] performed a structural study of UDD using X-ray diffraction and small angle X-ray scattering. They evidenced a diamond cluster core of about 43 A, the surface of which is covered by a mixture of sp2-bonded and sp3-bonded carbons. The authors could explain their small-angle X-ray scattering (SAXS) measurement with a model in which the diamond core is surrounded by onionlike carbon shells and nanosized graphite platelets. The thickness of these surrounding shells seems to be much dependent on the type of detonation synthesis (dry technology-gas cooling, or wet technology-water cooling). Baidakova et al.[20] further analyzed the fractal dimension of the external shells of nanodiamonds. On annealing, they show that the diamond content of the particles decreases from 1200 K in favor of the formation of graphite flakes on the surface of particles and in favor of onion shells at temperatures 1300 K and higher. Similar transformations occur under electron beam annealing as well. Fig. 1 shows the evolution of the electron energy loss spectroscopy (EELS) of a UDD nanoparticle as a function of exposure time. The spectra rapidly evolve from diamond-like to amorphous-like because of heating produced by the electron beam.
Other detonation-produced (TNT + RDX) nanodia-monds were analyzed by Chen et al.[21] In their study, the particles were almost perfectly spherical and 4-6 nm in diameter, but some larger 15 nm) spherical particles were also present. The composition analysis evidenced 87-90% carbon, 0.5-1% hydrogen, 1.6-2.5% nitrogen, and 6-10% oxygen. The authors explained the spherical shape of the particles as a consequence of the recrys-tallization of a liquidlike carbon droplet during the detonation. The structure was shown to be diamond, with a small proportion of sp2-bonded carbon atoms.
Fig. 1 Electron energy loss spectroscopy of a single nanodia-mond particle. (Courtesy of T. Van Buuren and J. Plitzko.) Left: HRTEM picture of the UDD powder. The particle under study (~ 3 nm in diameter) is designated by the circle. The reader can notice the enhanced atomic planes. Right: EELS spectra as a function of exposure time.
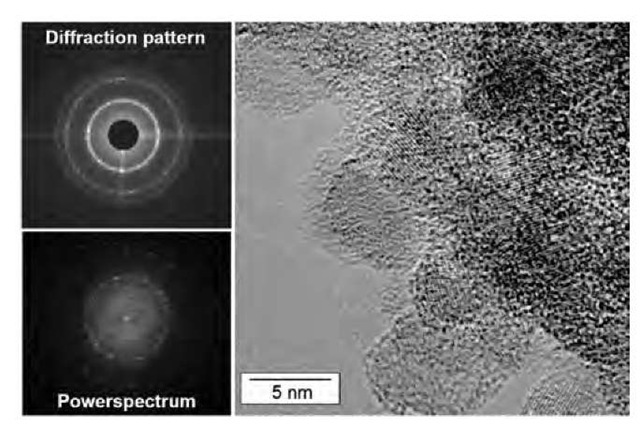
The small UDD nanocrystals often aggregate in larger conglomerates, as shown in the study of Aleksenskii et al.,[22] with small distortion of the initial spherical particle shape (Fig. 2).
The structure and defects of UDD have been extensively studied. In Ref. [24], infrared spectroscopy evidences O-H, C-H, C=C, C=0, and C-O-C groups. Other surface groups are evidenced by nuclear magnetic resonance (NMR). The electron spin resonance measurement indicates that nitrogen is not present as a substi-tutional (paramagnetic) site inside the particle’s core. However, another group[25] has measured a high concentration of paramagnetic centers. These are attributed to \dangling C—C bonds located at the interface between the diamond core and the graphene sheets forming the surface. A similar paramagnetism has been observed in Ref. [26].
Fig. 2 Electron diffraction pattern (top left), power spectrum (bottom left), and HRTEM picture of the edge zone of a conglomerate of nanodiamonds (UDD).
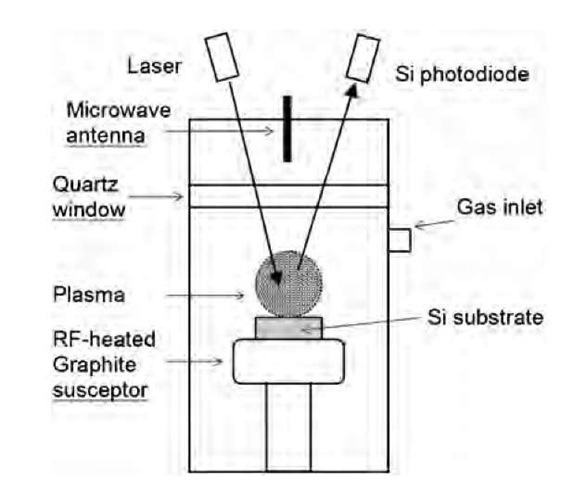
Fig. 3 Schematic representation of a plasma CVD reactor used in ultrananocrystalline diamond films production.
Nanodiamond CVD Production
In the process of optimizing the CVD technique for the generation of high-quality diamond films, it was shown that, under some conditions, the deposited film was no more a microcrystals assembly, but a smooth film of much smaller diamond particles (Fig. 3).
A typical CVD experiment would involve the injection of a mixture of methane and hydrogen gas in a plasma reactor. The diamond film, with variable morphologies, is deposited on a silicon substrate.[28] Under certain conditions of relative CH4/H2 concentrations and substrate temperature, it has been observed that the diamond film consisted of much smaller particles. The CVD-deposited diamonds films are distinguished into three categories: microcrystalline diamonds (0.5-10 mm), nanocrystalline diamonds (50-100 nm), and ultrananocrystalline (UNC) diamonds (2-5 nm). Garcia et al.[29] reported diamond particles of 50 nm diameter by applying a negative d.c. bias voltage during the first minutes of the deposition from a mixture of 4% CH4 in H2. The nanometric diamonds appear to be the first stage of diamond growth as long deposition times show them coalesce to form a micro-crystalline diamond film. Gruen et al. managed to optimize the gas concentration to generate UNC diamonds.a Replacing the major part of hydrogen by argon (typical 1% hydrogen) in the plasma causes the deposition of a smooth film of nanoparticles of only a few nanometers in size (Fig. 4).[30] Those UNC films contain only 2-5% of sp2-bonded carbons in grain boundaries and less than 1% hydrogen.
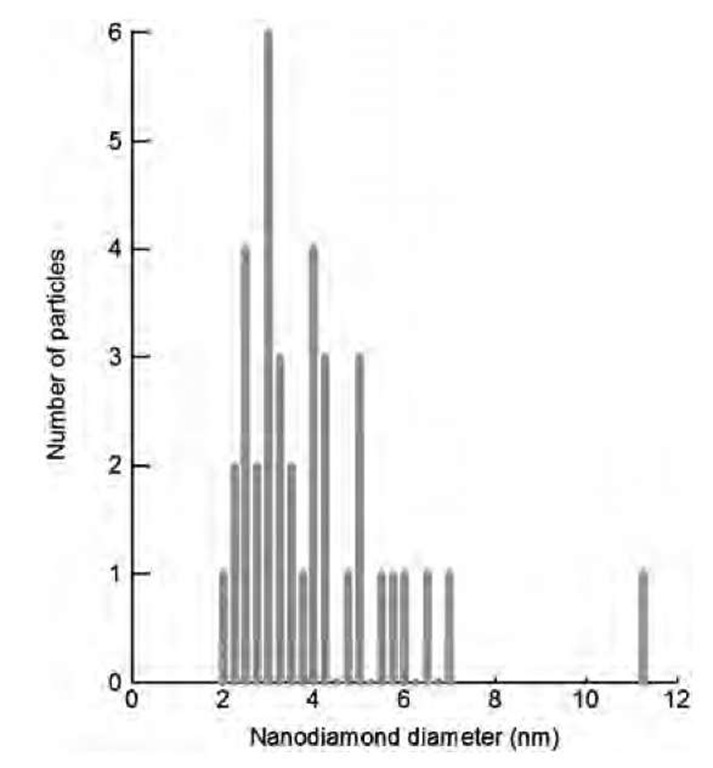
Fig. 4 Size histogram obtained from the analysis of an HRTEM image of an ultrananocrystalline diamond film where C60 was used as a substitute to CH4 in the CVD reactor.
Different plasma CVD techniques are used to produce diamond films. Nanocrystalline diamond crystals with a typical size of 50-100 nm are produced by hollow cathode arc plasma CVD, or direct current glow discharge-assisted CVD, among many other experiments.1-32-1 In that last study, a detailed analysis of the proportion of nanodia-monds to graphite shows that up to 75% of carbon is present in the film as sp3-bonded carbon in 3- to 5-nm diamond crystals. The remaining sp2-bonded atoms are mostly present in the form of a thin graphite layer (150200 nm) present between the substrate and the UNC diamond, as well as in grain boundaries. This UNC film has been shown to be stable up to 950°C.[33]