INTRODUCTION
Ceramics is one of the fields where nanoscience and nanotechnology have shown remarkable progress, producing a variety of advanced materials with unique properties and performance. Nanoceramics is a term used to refer to ceramic materials fabricated from ultrafine particles, i.e., less than 100 nm in diameter. In this field, a great deal of research has been accomplished in the last 20 years and has resulted in significant outcomes that are of great impact academically as well as industrially.
OVERVIEW
Advanced ceramics include inorganic and nonmetallic solid materials composed of polycrystalline sintered bodies, fine powders, single crystals, noncrystalline materials, thin or thick films, and fibers with various morphologies. Systems of metal oxides, carbides, borides and nitrides compose most of the important ceramic materials. Traditional ceramics, or old ceramics, such as tile pottery, are made from minerals such as clay; however, industrial ceramics, or advanced ceramics, are made of highly pure well-chosen materials such as silicon carbide and alumina. Many people think that ceramic materials are used for artistic objects and tableware only. In fact ceramic products are now very important in a wide range of industrial and advanced technical applications in several fields including electronics, medicine, nuclear industry, magnetic applications, and several others.
It has been well proven that the bulk behavior of materials can be dramatically altered when constituted of nanoscale building blocks. Mechanical, magnetic, optical, and other properties of materials have been found to be favorably affected. Hardness and strength, as an example, can be greatly enhanced by consolidating ceramic materials from nanoscale particles. Ductility and superplastic-forming capabilities of nanophase ceramics have now become possible, leading to new processing routes that will be more cost-effective than traditional methods.
In this article, our focus will be on advanced ceramics fabricated from nanometer-sized powders. Preparation, properties, and applications will be the main directions of focus and a special attention will be given to the effect of the particle size in these materials.
PREPARATION
Remarkable progress in synthetic chemistry has led to significant advances in material science, making possible the synthesis of various substances and materials. The manufacture of ceramics involves heat treatment of tightly squeezed powders. The size of the building block of these powders has been found to affect the properties of the final product. The method of preparation is very often a determining factor in shaping the material and its properties. For example, burning Mg in O2 (MgO smoke) yields 40-80-nm cubes and hexagonal plates, whereas thermal decomposition of commercial Mg(OH)2, MgCO3, and especially Mg(NO3)2 yields irregular shapes often exhibiting hexagonal platelets. Surface areas can range from 10 m2/g (MgO smoke) to 150 m2/g for Mg(OH)2 thermal decomposition. On the other hand, aerogel-prepared Mg(OH)2 can lead to MgO with surface areas as high as 500 m2/g.
Because the main focus of this article is ceramics fabricated from nanometer-sized building blocks, different methods for preparing ultrafine ceramic powders will be discussed. The steps in manufacturing ceramics from powders, which include molding, extrusion, and densifi-cation, will not be discussed here.
Physical Methods
Vapor condensation methods
Gas-condensation techniques to produce nanoparticles directly from a supersaturated vapor of metals are among the earliest methods for producing nanoparticles. They generally involve two steps: First, a metallic nanophase powder is condensed under inert convection gas after a supersaturated vapor of the metal is obtained inside a chamber. Second, the powder is oxidized by allowing oxygen into the chamber (to produce metal oxide powder). A subsequent annealing process at high temperatures is often required to complete the oxidation. The system consists of a vapor source inside a vacuum chamber containing a mixture of an inert gas, usually argon or helium, mixed with another gas, which is selected based on the material to be prepared. Oxygen is mixed with the inert gas to produce metal oxides. NH3 is usually used to prepare metal nitrides and an appropriate alkane or alkene, as a source of carbon, is usually used to prepare metal carbides. Nanoparticles are formed when supersaturation is achieved above the vapor source. A collection surface, usually cooled by liquid nitrogen, is placed above the source. The particles are transported to the surface by a convection current or by a combination of a forced gas flow and a convection current, which is set up by the difference in the temperature between the source and the cold surface. Some improved systems involve a way to scrap the nanoparticles from the cold collection surface so that the particles would fall into a die and a unit where they can be consolidated into pellets. Supersaturated vapor can be achieved by many different vaporization methods. The most common techniques include thermal evaporation, sputtering, and laser methods. A variety of nanoscale metal oxides and metal carbides have been prepared using laser-vaporization techniques.
The advantages of vapor condensation methods include versatility, ease in performance and analysis, and high-purity products. On the other hand, they can be employed to produce films and coatings. Furthermore, laser-vaporization techniques allow for the production of high-density, directional, and high-speed vapor of any metal within an extremely short time. Despite the success of these methods, they have the disadvantage that the production cost is still high because of low yields. Heating techniques have other disadvantages that include the possibility of reactions between the metal vapors and the heating source materials.
Spray pyrolysis
This technique is known by several other names including solution aerosol thermolysis, evaporative decomposition of solutions, plasma vaporization of solutions, and aerosol decomposition. The starting materials in this process are chemical precursors, usually appropriate salts, in solution, sol, or suspension. The process involves the generation of aerosol droplets by nebulizing or ”atomization” of the starting solution, sol, or suspension. The generated droplets undergo evaporation and solute condensation within the droplet, drying, thermolysis of the precipitate particle at higher temperature to form a microporous particle, and, finally, sintering to form a dense particle.
Different techniques for atomization are employed including pressure, two-fluid, electrostatic, and ultrasonic atomizers. These atomizers differ in droplet size (2-15 mm), rate of atomization, and droplet velocity (1-20 m/sec).
These factors affect the heating rate and residence time of the droplet during spray pyrolysis which, in turn, affect some of the particle characteristics including particle size. For a specific atomizer, particle characteristics, including particle size distribution, homogeneity, and phase composition depend on the type of precursor, solution concentration, pH, viscosity, and the surface tension.
Aqueous solutions are usually used because of their low cost, safety, and the availability of a wide range of water-soluble salts. Metal chloride and nitrate salts are commonly used as precursors because of their high solubility. Precursors that have low solubility or those that may induce impurities, such as acetates that lead to carbon in the products, are not preferred.
The advantages of this method include the production of high-purity nanosized particles, homogeneity of the particles as a result of the homogeneity of the original solution, and the fact that each droplet/particle goes through the same reaction conditions. The disadvantages of spray pyrolysis include the need for large amounts of solvents and the difficulty to scale-up the production. The use of large amounts of nonaqueous solvents increases the production expenses because of the high cost of pure solvents and the need for proper disposal.
Thermochemical/flame decomposition of metalorganic precursors
Flame processes have been widely used to synthesize nanometer-sized particles of ceramic materials. This is another type of gas-condensation technique with the starting material being a liquid chemical precursor. The process is referred to as chemical vapor condensation (CVC). In this process, chemical precursors are vaporized and then oxidized in a combustion process using a fuel-oxidant mixture such as propane-oxygen or methane-air. It combines the rapid thermal decomposition of a precursor-carrier gas stream in a reduced pressure environment with thermophoretically driven deposition of the rapidly condensed product particles on a cold substrate. The flame usually provides a high temperature (1200-3000 K), which promotes rapid gas-phase chemical reactions.
A variety of chemical precursors can be used including metal chlorides, such as TiCl4 to prepare TiO2 and SiCl4 to prepare SiO2, metal-alkyl precursors, metal alkoxides, and gaseous metal hydrides, such as silane as a source of silicon to prepare silica. Chlorides have been the most widely used precursors in the industry and the process is sometimes referred to as the ”chloride process.” The high vapor pressure of chlorides and the fact that they can be safely stored and handled make them excellent potential precursors. The disadvantages of using chloride precursors are the formation of acidic gases and contamination of the products with halide residues. Flame processes are used industrially to produce commercial quantities of ceramic particulates, such as silica and titania. This is because of the low cost of production as compared to all other methods. The disadvantage of flame synthesis is that the control of particle size (both primary particle and aggregates size), morphology, and phase composition is difficult and limited.
Chemical Methods
Sol-gel technique
The sol-gel process is typically used to prepare nanometer-sized particles of metal oxides. This process is based on the hydrolysis of metal reactive precursors, usually alkoxides in an alcoholic solution, resulting in the corresponding hydroxide. Condensation of the hydroxide by giving off water leads to the formation of a network-like structure. When all hydroxide species are linked, gelation is achieved and a dense porous gel is obtained. The gel is a polymer of a three-dimensional skeleton surrounding interconnected pores. Removal of the solvents and appropriate drying of the gel result in an ultrafine powder of the metal hydroxide. Further heat treatment of the hydroxide leads to the corresponding powder of the metal oxide. As the process starts with a nanosized unit and undergoes reactions on the nanometer scale, it results in nanometer-sized powders. For alkoxides that have low rates of hydrolysis, acid or base catalysts can be used to enhance the process.
When drying is achieved by evaporation under normal conditions, the gel network shrinks as a result of capillary pressure that occurs and the hydroxide product obtained is referred to as xerogel. However, if supercritical drying is applied using a high-pressure autoclave reactor at temperatures higher than the critical temperatures of solvents, less shrinkage of the gel network occurs as there is no capillary pressure and no liquid-vapor interface, which better protects the porous structure. The hydroxide product obtained is referred to as an aerogel. Aerogel powders usually demonstrate higher porosities and larger specific surface areas than analogous xerogel powders.
Sol-gel processes have several advantages over other techniques to synthesize nanopowders of metal oxide ceramics. These include the production of ultrafine porous powders and the homogeneity of the product as a result of homogeneous mixing of the starting materials on the molecular level.
Reverse microemulsions/micelles method
The reverse micelle approach is one of the recent promising routes to nanocrystalline materials including ceramics. Surfactants dissolved in organic solvents form spheroidal aggregates called reverse (or inverse) micelles. In the presence of water, the polar head groups of the surfactant molecules organize themselves around small water pools 100 A), leading to dispersion of the aqueous phase in the continuous oil phase.
Reverse micelles are used to prepare nanoparticles by using a water solution of reactive precursors that can be converted to insoluble nanoparticles. Nanoparticle synthesis inside the micelles can be achieved by different methods including hydrolysis of reactive precursors, such as alkoxides, and precipitation reactions of metal salts. Solvent removal and subsequent calcination lead to the final product. Several parameters, such as the concentration of the reactive precursor in the micelle and the weight percentage of the aqueous phase in the microemulsion, affect the properties, including particle size, particle-size distribution, agglomerate size, and the phases of the final ceramic powders. There are several advantages to using this method including the ability to prepare very small particles and the ability to control the particle size. Disadvantages include low production yields and the need to use large amounts of liquids.
Precipitation from solutions
Precipitation is one of the conventional methods to prepare nanoparticles of metal oxide ceramics. This process involves dissolving a salt precursor, usually chloride, oxy-chloride or nitrate, such as AlCl3 to make Al2O3, Y(NO3)3 to make Y2O3, and ZrCl4 to make ZrO2, in water. The corresponding metal hydroxides are usually obtained as precipitates in water by adding a base solution such as sodium hydroxide or ammonium hydroxide solution. The remaining counter-ions are then washed away and the hydroxide is calcined after filtration and washing to obtain the final oxide powder. This method is useful in preparing ceramic composites of different oxides by co-precipitation of the corresponding hydroxides in the same solution. Solution chemistry is also used to prepare non-oxide ceramics or pre-ceramic precursors that can be converted to ceramics upon pyrolysis.
One of the disadvantages of this method is the difficulty in controlling the particle size and size distribution. Very often, fast and uncontrolled precipitation takes place resulting in large particles.
Chemical synthesis of pre-ceramic polymers coupled with physical processing techniques
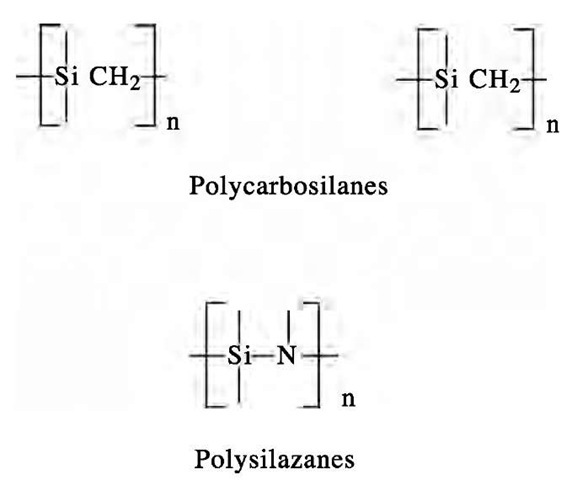
This method is based on the use of molecular precursors, which facilitates the synthesis of nanomaterials containing phases of desired compositions. It involves a chemical reaction to prepare an appropriate polymer, which is then converted into ceramic material upon pyrolysis. Using chemical reactions to prepare the pre-ceramic polymer not only allows for control of phase compositions but also overcomes the limitation of low production yields of the physical methods. This method has been very useful in preparing nonoxide ceramics such as silicon carbide and silicon nitride. The conversion of an organometallic precursor into a ceramic depends on different parameters such as the molecular structure of the precursor and the pyrolysis conditions (temperature, duration, and atmosphere). Metal carbides and metal nitrides have been obtained by pyrolysis of polymers containing the appropriate elements such as Si or Al and C or N. These polymers are called pre-ceramic polymers and are prepared from simpler chemical precursors. A considerable amount of free carbon from the thermolysis process is very often a problem. Silicon carbide (SiC) and silicon nitride (Si3N4) are the most studied ceramic materials prepared via this route. They are usually synthesized by the pyrolysis of polycarbosilanes and polysilazanes, for which general structural formulas are shown in Fig. 1, at temperatures between 1000°C and 1200°C.
Fig. 1 General structural formulas of polycarbosilanes and polysilazanes.
Mechanochemical synthesis
Mechanochemical synthesis involves mechanical activation of solid-state displacement reactions. This process has been successfully used recently to make nanoceramic powders such as Al2O3 and ZrO2. It involves the milling of precursor powders (usually a salt and a metal oxide) to form a nanoscale composite of the starting materials,which react during milling, and subsequent heating, if necessary, to form a mixture of dispersed nanoparticles of the desired oxide within a soluble salt matrix. Nanopar-ticles of Al2O3 (10-20 nm), for example, can be prepared by milling AlCl3 with CaO.
BONDING CONSIDERATIONS
Understanding chemical bonding and structures in ceramic materials is necessary in order to understand their chemical and physical behavior. When materials are composed of nanometer-sized building blocks, they deviate considerably from structural perfection and stoichiometry. As a result, the number of defects due to edges, corners, f-centers, and other surface imperfections is greatly enhanced, which, in turn, affects several physical and chemical properties as will be discussed below.
A range of cohesive forces contribute to the nature of bonding in ceramic materials including ionic (MgO, Fej _xO), covalent, metallic, van der Waals, and hydrogen bonding. Ionic compounds are formed when highly electronegative and highly electropositive elements are combined in a lattice. Pure ionic model is a reasonable approximation for some systems while it is a poor approximation for crystals containing large anions and small cations. In such systems, covalent contribution to bonding becomes significant. Van der Waals interactions play a crucial role in many ceramic systems, especially those with layered structures. In many oxide hydrates or hydroxy oxides, hydrogen bonding also contributes to the cohesive energy. For further reading on structure and bonding, the reader is referred to structural inorganic chemistry books.
SELECTED PROPERTIES
Ceramics possess their own chemical, physical, mechanical, and magnetic properties that are different from those of other materials such as metals and plastics. The properties of ceramics depend mainly on the type and the amounts of materials in their composition. However, the size of the building blocks of a ceramic material has been found to play an important role in its properties (see Ref. [1] and references therein).
When materials are prepared from nanometer-sized particles, a significant portion of the atoms become exposed on the surface. As a result, such materials exhibit unique properties that are remarkably different from those of the corresponding bulk. The physical and chemical properties of nanoparticles show the gradual transition from atomic or molecular to condensed matter systems.
Chemical Properties
Ceramic materials are relatively inert, especially crystalline materials that tend to have perfect structures with minimum amount of defects. Most of the reactivity of these materials involves the surfaces where coordinatively unsaturated as well as defect sites exist. The behavior of the surface toward other species and the nature of interaction depend on the composition and the morphology, which determine the nature and the degree of surface interactions with other substances. Most of the time, interactions are limited to adsorption on the surface, which does not affect the bulk making these materials good corrosion-resistant.
The possibility of preparing ceramic powders in high surface areas with high porosity makes them well desired in some advanced applications. One example is the use of ceramic materials as supports for heterogeneous catalysts. Another example is the use of such materials in biomedical applications, where the surface of nanophase ceramics exhibits a remarkably improved biomedical compatibility compared to conventional ceramics, as discussed below.
Mechanical Properties
Ceramics are very strong materials showing considerable resistance against compression and bending. Some ceramic materials are similar to steel in strength. Most ceramics retain their strength at high temperatures. Silicon carbides and silicon nitrides, as an example, retain their strength at temperatures as high as 1400°C. As a result, such materials are used in high-temperature applications. Many of the physical and mechanical properties are particle-size dependent. As a result, several systems of nanophase ceramics have exhibited quite interesting and favorably enhanced mechanical properties.
Improved sintering and hardness properties
Nanoceramics are processed from nanophase powders by compacting first powders composed of individual ceramic particles (usually less than 50 nm in size) into a raw shape (often called a green body). This compacted powder is then heated at elevated temperatures. Densification occurs as a result of diffusion of vacancies out of pores (to grain boundaries), which lead to shrinkage of the sample. This process is referred to as pressure-less sintering. Fortunately, nanophase powders were found to compact as easily as their analogous submicron particles. To avoid particle size growth, samples have to be sintered at the lowest temperature possible for a time sufficient to remove the residual porosity and establish coherent grain boundaries. Successful sintering enhances the hardness of the final material.
Experimental evidence shows that nanophase powders densify at faster rates as compared to commercial (submicron) particles.[1] Faster densification rates allow achieving a given density at smaller grain sizes, before serious growth takes place. As a result of their small particle and pore sizes, nanocrystalline powders sinter to much greater densities than their conventional analogs at the same temperature. This also establishes that nanocrys-talline powders, as compared to conventional powders, reach the same density at much lower temperatures. This, of course, eliminates the need for very high temperatures.
One disadvantage that can accompany fast densifica-tion though is inhomogeneous heating where the outside layers of the particles densify into a hard impervious shell which constrains the inside of the sample from normal shrinking, leading to some cracking as a result of strain incompatibility. This problem can be avoided by several ways. The most efficient way is to heat the samples slowly to reduce the shrinkage in the outer shell while heat is transported to the inner regions. On the other hand, high-density nanostructured ceramic systems including Y2O3, TiO2, and ZrO2 have been achieved by means of pressure-assisted sintering. Applying some pressure during sintering can increase the densification rate and suppress the particle growth.
Nanoscale powders of nonoxide ceramics such as metal carbides and nitrides show similar behavior. Conventional SiC, as an example, is difficult to sinter. Addition of some additives such as boron or carbon is very often necessary to densify SiC. Ultrafine powder of SiC sinters at lower temperatures and densifies without additives. On the other hand, mechanical properties can be fairly improved by the introduction of metallic nanoparticles dispersed within the matrix grains. Such systems are referred to as nanocomposites. Tungsten, nickel, or molybdenum nano-particles dispersed within Al2O3 matrix grains, as an example, can enhance the mechanical properties of alumina, including the fracture strength and hardness.
Reduced brittleness and enhanced ductility and superplasticity
Superplasticity and ductility refer to the capability of some polycrystalline materials to undergo extensive tensile deformation without necking or fracture. Ceramic brittleness is the biggest technical barrier in practical applications, especially in load-bearing applications. Theoretical and experimental results provide evidence for the possibility that traditional brittle materials can be ductilized by reducing their grain sizes.[1] When made from nanoparticles, brittle ceramics can be superplastically deformed at modest temperatures and then heat treated at higher temperatures for high-temperature strengthening.
The capability to synthesize superplastic ceramic materials is now established. Nanocrystalline ceramics deform at faster rates, lower stresses, and lower temperatures. One important use of superplasticity in ceramics is diffusion bonding, where two ceramic parts are pressed together at moderate temperatures and pressures to form a seamless bond through diffusion and grain growth across the interface. Diffusion bonds form more easily in nanocrystalline ceramics than in larger grained ceramics as a result of both the enhanced plastic flow of nanocrys-talline ceramics and the larger number of grain boundaries they provide for diffusional flux across the interface.
Electrical Properties
Ceramics include electrical conducting, insulating, and semiconducting materials. Chromium oxide is an electrical conductor, aluminum oxide is an insulator, while silicon carbide behaves as a semiconductor. As a result, ceramic materials have been used in a variety of electronic applications based on their electrical behavior.
Several electrical properties are particle-size and composition dependent. Electrical resistance and dielectric constant, as an example, for some systems increased as a result of small particle size. Conductivity of some mixed oxide ceramics, such as lithium aluminosilicate, is higher than that of their constituent oxides.
Magnetic Properties
Some ceramic materials possess magnetic properties. These include iron oxide-based ceramics and oxides of chromium, nickel, manganese, and barium. Ceramic magnets are known to exhibit high resistance to demagnetization. As a result, several ceramic powders have been employed in a wide range of electronic and magnetic applications as discussed below.
The fabrication of such materials from ultrafine particles can significantly enhance their magnetic behavior. The fact that in nanometer-sized particles a large portion of the atoms are on the surface, where the coordination numbers are less than that for bulk atoms, affects several parameters including unique surface/interface behavior and different band structure, which both lead to magnetism enhancement. It is now well established that one of the requirements to achieve appropriate coercivity and high magnetization saturation is to fabricate such materials in highly divided particles, preferably in the nanometer-sized range, with homogeneity and narrow size distribution.
Many other properties are also particle-size dependent. The optical properties, as an example, of some ceramic materials have been found to depend on particle sizes. Nanoparticles of TiO2, as an example, are more efficient UV absorber than powders of large particles.
APPLICATIONS
Ceramic materials are of great value in a variety of applications as a result of their unique properties compared to other materials. Because of their electrical and magnetic properties, ceramics are important in several electronic applications, where they are used as insulators, semiconductors, conductors, and magnets. Ceramic materials also have important uses in the aerospace, bio-medical, construction, and nuclear industries. In many of these applications, ceramic materials have shown significantly better performance when fabricated from nanometer- sized particles.
Mechanical Applications
Industrial ceramics are widely employed in applications that require strong, hard, and abrasion-resistant materials. Metal-cutting tools, tipped with alumina, and tools made from silicon nitrides for cutting, shaping, grinding, and sanding iron, nickel-based alloys, and other metals are very commonly used. Other ceramics such as silicon nitrides and carbides are used to make components for high-temperature use such as valves and turbocharger rotors. Ceramic materials and metal-based ceramics (cermets) are used to make components for space vehicles, including heat-shield tiles for the space shuttle and nosecones for rocket payloads.
Electrical Applications
Ceramics are used as insulators, semiconductors, and conductors. Aluminum oxide (Al2O3), for example, does not conduct electricity at all and is used to make insulators. Other ceramics, such as barium titanate (BaTiO3), are used as semiconductors in electronic devices. Some copper oxide-based ceramics are superconductive at temperatures higher than those at which metals become superconductive. Superconductivity refers to the ability of a cooled material to conduct an electric current with no resistance. This phenomenon can occur only at extremely low temperatures, which are difficult to maintain. Transition metal nitrides, carbides, and borides are of interest as cathodes in electrochemical applications. This interest stems from the favorable properties of these materials including electronic conductivity and good thermal conductivity, which when coupled with their mechanical strength and high melting points suggest that such materials can be stable in a range of environments.
Table 1 Examples of electronic ceramics
|
Functions |
Examples of materials |
Applications |
|
Insulation |
Al2O3, SiC + BeO |
IC substrate |
|
Dielectricity |
BaTiO3 |
capacitor |
|
Semiconducting |
SiC, LaCrO3, SnO2, ZnO + Bi2O3 |
gas defector, thermistor, varistor |
|
Piezoelectricity |
ZnO, SiO2 |
piezolighter, piezofilter, surfacewave transducer, |
|
piezovibrator, flexible piezodetector |
||
|
Pyroelectricity |
PZT |
IR detectors |
|
Ferroelectricity |
PLZT |
optical shutter, optical memory |
|
Ionic conduction |
b-Al2O3, ZrO2 |
Na-S battery, O2 sensor |
|
Luminescence |
Y2O2S:Eu, ThO2:Nd, Al2O3:Cr |
cathode luminescence, IR laser |
|
Light guide |
SiO2 |
optical communication fiber |
|
Polarization |
PLZT |
optical shutter |
|
Soft magnetism |
g-Fe2O3, Zn1 _xMnxFe2O4 |
magnetic tape |
|
Hard magnetism |
SrO ■ 6Fe2O3 |
magnet seal |
Some ceramics such as strontium titanate (SrTiO3) are employed in the form of thin films as capacitors in several electronic devices because of their capability to store large amounts of electricity in extremely small volumes. Lithium aluminosilicate ceramics have potential applications as solid electrolytes for utilization in solid high-energy density lithium battery systems. Piezoelectric ceramics are now key electronic components for television, FM radio, and the like. Very recently, piezoelectric ceramic displays have been developed in Japan, where microceramic actuators for activating the pixels are used. Piezoelectric effect refers to the appearance of an electric potential across certain faces of a crystal when it is subjected to mechanical pressure.
Other examples of functions and applications of advanced ceramics in the field of electronics are shown in Table 1.[2]
Magnetic Applications
Iron oxide-based ceramics (ferrites) are widely employed as low-cost magnets in electric motors. Such magnets help in converting electric energy into mechanical energy. Unlike metal magnets, ferrites conduct high-frequency currents, and as a result, they do not lose as much power as metal conductors do. Manganese zinc ferrites are used in magnetic recording heads, and ferric oxides are the active component in several magnetic recording media, such as recording tapes and computer diskettes.
Biomedical Applications and Bioceramics
Some advanced materials are used in the biomedical field to make implants for use within the body. The main requirement of biomaterials for this application is the ability of their surfaces to support new bone growth. Ceramic materials are known to possess exceptional biocompatibil-ity properties with bone cells and tissues. Specially made porous ceramic materials such as alumina, titania, zirconia, and others bind with bone and other natural tissues. Such ceramics are used to make hip joints, dental caps, and bridges. Other advanced ceramics such as hydroxyapatite, Ca10(PO4)6(OH)2, which is the principal component of bones and teeth, have excellent biocompatibility and bone in-growth capabilities and are used in reconstructing fractured bones and as replacement materials.
Recent research on long-term functions of osteoblasts on nanophase ceramics has shown evidence for unique and significant behavior.[3] Compared to conventional ceramics, nanophase ceramics has shown enhanced osteoblast adhesion and proliferation, alkaline phosphatase synthesis, and concentration of extracellular matrix calcium.
Coatings
Because of its unique hardness and corrosion resistance, ceramic enamel is often used in coating metals. Thin hard wear-resistant coatings of ceramics include materials such as titanium nitride and titanium carbonitride. An emerging class of new hard protecting coatings beyond homogeneous layers of a ceramic nitride material is layered coating structures such as superlattices or multilayers of different nitrides. Such multilayer coating has been successfully applied in several applications such as bearings,pumps, and compressors. Nonnitride coatings, such tungsten carbide/carbon, are also of interest because of their high elasticity and chemical inertness.
Several techniques are being employed for protective ceramic coating. These include thermal spraying, chemical vapor deposition (known as CVD), and plasma spraying.
Nuclear Industry
Lithium-based ceramics are now considered as potential solid tritium breeders in nuclear fusion reactors. Potential breeder materials include LiAlO2, Li2O, Li2ZrO3, and Li4SiO4. Solid breeders are safer during operation than liquid lithium systems, which are highly reactive.
CONCLUSION
Nanoceramics is one of the great outcomes of the evolutionary research in the field of nanoscience and nanotechnology, where fabrication of materials from nanometer-sized building blocks has resulted in a wide range of industrially useful materials. Recent research has proven that ceramic materials fabricated from ultrafine powders can be obtained through several physical as well as chemical methods that can be scaled up to produce commercial amounts. These unique materials have exhibited very remarkable behavior as compared with their bulk counterparts. Significant characteristics include chemical, mechanical, magnetic, electrical, and optical properties. As a result, improved performance of ceramic materials has been observed in a variety of applications including chemical, mechanical, magnetic, electrical, and biomed-ical. The new properties and improved performance of nanoceramics that are being discovered stimulate the development and improvement of ceramic processing, which, in turn, open the doors wide for the use of ceramics in a wide range of new technologies.
Directions of current interests include the preparation and processing of ultrafine (nanometer-sized) powders, the development of new synthetic routes to materials of homogeneous sintered bodies, and the preparation of ceramics made of several composites.