EXPERIMENTAL EXAMINATION OF QUARTZ CRYSTAL MICROBALANCE THEORY AND ADSORPTION OF CO2 ON METAL SURFACES
As the frequency of a QCM crystal exposed to a high-pressure fluid is affected by different factors, it is critical to accurately separate out each influencing factor. Experimental verification of QCM theory enables the calculation of the actual mass change on the crystal surface. The investigation of CO2 adsorption on metal electrodes of blank crystals is a required initial step for the successful use of the QCM technique to investigate the chemical and physical properties of surface films in scCO2.
Separation of Contributions to Frequency
As the microscopic properties of crystal-fluid interfaces complicate the QCM frequency behavior, a unique strategy[40] to verify the QCM theory in scCO2 is to experimentally establish a relationship between the microscopic properties of crystal surfaces and frequency shifts. In a study reported here, the surface roughness of QCM crystals (three gold and three silver 5-MHz crystals; Table 3) was characterized using an AFM technique. Three groups of QCM experiments were performed on the six crystals to separate the following different contributions to QCM frequency: AFP, AFm, AFZ, and AFr. The first set of experiments performed on Ag-polished and Ag-rough crystals utilized helium, the lightest inert gas considered nonabsorbing (AFm=0) on metals, to determine AFP and AFZ. To evaluate the roughness contribution of frequency, AFr, the second set of experiments utilized N2, another nonabsorbing gas on the metal surfaces at room temperatures (i.e., AFm negligibly small). The last group of experiments was conducted using low-density gaseous CO2 (<0.2 g cm"3), an adsorbing gas at room temperatures, to test the adsorbed masses of CO2 on the metal surfaces.
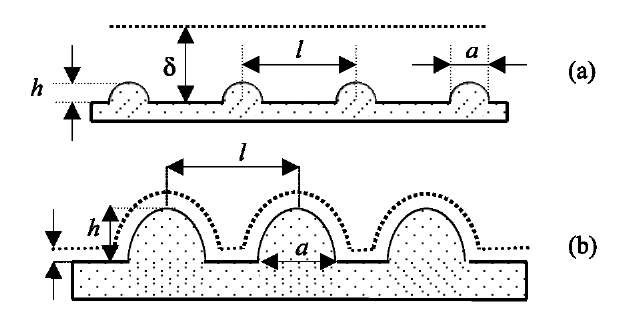
Fig. 5 Two types of ideal surface roughness. (a) Slightly rough, h/d < 1; (b) strongly rough, h/d > 1.
The first set of experiments in helium indicated that the frequency shifts of the smoothest silver crystal (Ag-polished) could be accurately predicted with the QCM theory for pressure and viscosity (Eqs. 3 and 4) by neglecting the roughness effect. However, for the roughest silver crystal (Ag-rough), about 10% of the total frequency shifts could not be theoretically predicted with Eqs. 3 and 4. The variations in the prediction were attributed to the roughness effect. This is consistent with the experimental findings by Tsionsky et al.[30] who investigated frequency shifts of gold and nickel crystals in He, H2, Ar, and N2.
The second set of experiments in nitrogen showed that the roughness effect had a significant influence on the frequency even for the smoothest silver and gold crystals. The experimental roughness contribution, AFr, was calculated by subtracting theoretical AFP and AFZ from the experimental AF (Eq. 1). The resulting AFr was plotted as a function of theoretical N2 density; the corresponding frequency shift corrected in this manner was found to be a nearly linear function of density, indicating the precision of the theoretical expression of AFr (Eq. 7). Therefore the frequency-roughness correlation factor, Cr, can be obtained by correlating AFr with the N2 density (f or the decay length (d), using Eqs. 8a and 8b,
where a and b are the constants assumed to be independent of the type of fluid. The values of a and b for each crystal are also shown in Table 3. Eqs. 8a and 8b represent accurate predictions of Cr with most correlation coefficients better than 0.999. Eq. 8a is only applicable to gases because of the neglect of the influence of viscosity, while
Table 3 Some characteristic properties of six different QCM crystals
|
|
|
|
|
Cr (10 |
5 cm)b |
|
|
|
RMS roughnessa |
H (10- 7 cm) |
a (10- 7 cm) |
l (10-7 cm) |
||
|
Crystals |
(10- 7 cm) |
Eq. 8a |
Eq. 8b |
|||
|
Ag-polished |
3.7 |
12 |
180 |
190 |
1.32(1-2.65pf) |
0.398+4.96 x 104d |
|
Ag-unpolished |
26 |
85 |
1410 |
2180 |
1.79(1-3.48pf) |
0.322 + 7.44 x 104d |
|
Ag-roughc |
647 |
1030 |
- |
- |
7.28(1-1.27pf) |
5.16 + 1.05 x 105d |
|
Au-polished |
2.8 |
8 |
110 |
120 |
1.37(1-2.23pf) |
0.595+4.10 x 104d |
|
Au-unpolished |
115 |
210 |
1370 |
2120 |
3.22(1-2.37pf) |
1.41+9.17 x 104d |
|
Au-roughc |
681 |
1090 |
- |
- |
6.88(1-1.51pf) |
3.99 + 1.63 x 105d |
Eq. 8a is suitable for dense gases (pf>0.2 g/cm3) and liquids.[40]Cr was found to be dependent primarily on the rms roughness of crystals and, to a lesser degree, on a and 1.[40]
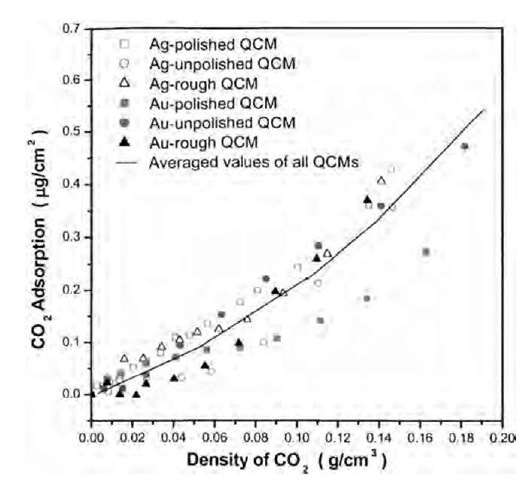
In the last group of experiments, the frequency shifts (DF) of all six crystals in gaseous CO2 (pressure <1000 psi) were measured to evaluate the amount of CO2 adsorbed on the metal surfaces. The CO2 adsorption was calculated from DFm that was obtained by subtracting the theoretical DFP, DFZ, and DFr from DF, using Eqs. 1 to 4, 7, and 8a. As can be seen (Fig. 6), all six crystals with different surface roughness values adsorbed similar amounts of gaseous CO2 per real surface area, which agrees well with the adsorption theory. This is additional evidence of the accuracy of the reformulated QCM theory by including the roughness contribution. It also implies that the a and b values obtained in N2 are independent of the type of fluid.
Adsorption of Supercritical CO2 on Metal Surfaces
Several researchers have determined the mechanisms of adsorption of scCO2 on metal surfaces. Otake et al.[41] determined the mass of CO2 adsorbed onto rough silver QCM electrodes at 40°C and at pressures up to 5800 psi. They concluded that scCO2 mass adsorption on metal surfaces could approach values as high as 25 mg/cm2. Guigard et al.[42] evaluated the adsorption of CO2 on rough gold electrodes at 40°C and 45°C and at pressures up to 1500 psi. They found the similar maximum amount of CO2 adsorption as by Otake et al. However, these researchers all neglected the roughness contribution to frequency in the calculation. In order to fully account for the roughness contribution, the Wu research group examined in greater detail the scCO2 adsorption on metal surfaces.[40]
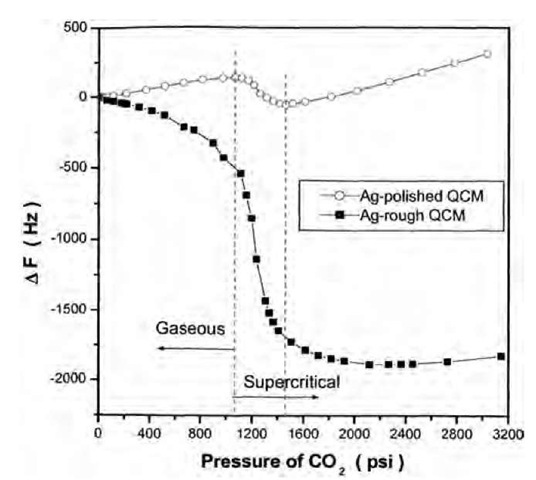
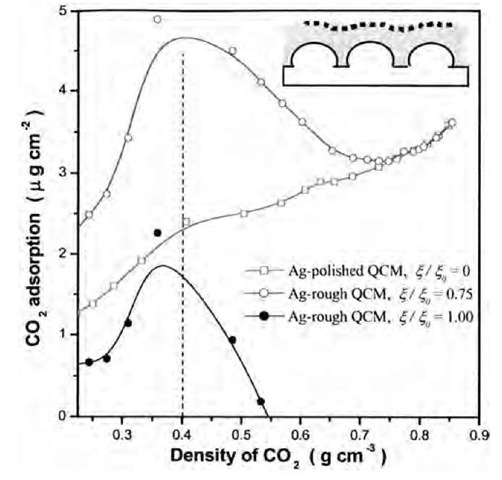
The frequency shifts of Ag-polished and Ag-rough crystals (Fig. 7) in scCO2 were measured to evaluate the amount of CO2 adsorbed on the surfaces (Fig. 8). The calculation procedure is the same as in the gaseous CO2. As the CO2 density and the amount of adsorbed CO2 increase with increasing pressure, the holes and valleys between the inhomogeneities on the crystal surface are gradually filled (Fig. 8, inset), which causes a gradual decrease of surface roughness.[36] Therefore Cr was corrected for the overestimation of the roughness contribution,1-40-1
Fig. 6 Adsorption of gaseous CO2 on silver and gold surfaces at 40°C, using the Cr values calculated with Eq. 8b.
where X and X0 are the roughness in centimeters of the original substrate surface and the modified surface by the absorbed molecules, respectively. X is a function of thickness (or mass) of absorbed molecular layers. Analysis showed that it was appropriate to predict scCO2 adsorption on the Ag-polished crystal by neglecting the roughness contribution (Cr=0 or X = 0), when the density was larger than 0.4 g cm"3 (Fig. 8). This was because the quantity (2.4 mg/cm2) of CO2 mass absorbed at the density of 0.4 g cm" 3 could smooth the surface of Ag-polished crystal.[40] For the Ag-rough crystal, calculation showed that the two limits of £/£0= 1.0 and 0.75 could fit the adsorption data of the Ag-polished crystal well in the regions of low and high densities (<0.4 and >0.7 g/cm3; Fig. 8). It was concluded that the use of rough QCM crystals (rms roughness >10 nm) required careful attention in scCO2 adsorption studies, because of the variation of Cr.[40] Only very smooth QCM crystals (several nanometers or less) are capable of accurately determining the adsorption of scCO2 on the metal surfaces without considering the surface roughness contribution. The adsorption of scCO2 on the silver and gold surfaces at 40°C ranges to 3.6 mg/cm2, but not up to 25 mg/cm2 as determined by Otake et al.[41] and Guigard et al.[42] who used very rough crystals and neglected the roughness contribution.
Fig. 7 Frequency shift of Ag-polished and Ag-rough crystals in gaseous and scCO2 at 40°C as a function of pressure.
APPLICATIONS OF QUARTZ CRYSTAL MICROBALANCE FOR DISSOLUTION STUDY OF POLYMER FILMS IN SUPERCRITICAL CO2
Polymer dissolution (or polymer desorption) in solvents is an important phenomena in polymer science and engineering. Applications of polymer dissolution or desorption are found in several areas such as microlithography, controlled drug delivery, and plastics recycling.[43] For example, the successful implementation of a microlithog-raphy process requires technical information on the dissolution of photoresist polymer films in specific solvents during 1) the development of the resist images and 2) the stripping of the remaining films after the lithography process.[44] Hinsberg et al.[45,46] and Ito[47] applied the QCM techniques to determine the dissolution rate of novolac and poly(4-hydroxystyrene) films in aqueous developer solutions at atmospheric pressure. Mueller et al.[48] have used the QCM to investigate the diffusivity of casting organic solvents in photoresist films for the evaluation of the influence of the solvents on the polymer dissolution behavior. These researchers demonstrated that the QCM technique was a particular attractive tool for the evaluation of the dissolution of polymer films in organic and aqueous solutions.
The dissolution kinetics of polymer films in scCO2 provides useful technical information for the development of scCO2-based interfacial processes that require excellent control of surface properties (e.g., thickness and morphology) at the micro- and nanometer scales. The recently proposed surface processing of microelectronics in scCO2[9] is a typical field that requires information on polymer film dissolution. However, a search of the literature in this specific area revealed that there have been a very limited number of studies on the polymer dissolution kinetics in scCO2 by the use of QCM technique. To date, the number of dissolution studies of polymer films in scCO2 by other online techniques, e.g., interferometry,[49] is also limited. This is probably due to the lack of various detection techniques at extreme conditions and to the situation of such scCO2-based processes being in the stages of early development. Nevertheless, Wu and Grant[50] have utilized the QCM for the evaluation of polymer film dissolution in scCO2.
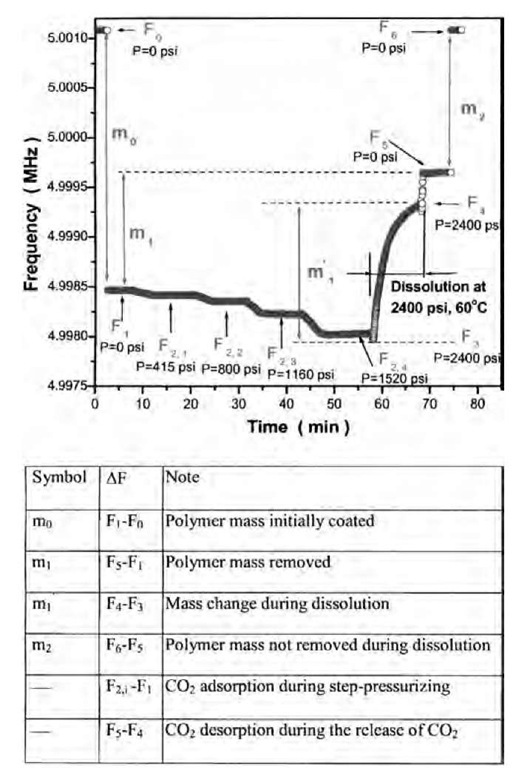
Based on the availability and applicability of the reformulated QCM theory in scCO2, the dissolution study of polymer films in scCO2 was achieved utilizing the experimental high-pressure QCM apparatus (Fig. 4). Fig. 9 represents a typical experimental run using a Si-polished QCM crystal to evaluate the dissolution kinetics of poly (FOMA-r-THPMA) films (low MW polymer) at 60°C and 2400 psi. The fundamental frequency (F0; Fig. 9) of the blank crystal was determined in vacuum (P=0 psi). The crystal was removed from the vessel and the polymer film was cast onto the crystal by dip-coating in an 8 wt.% polymer-trifluorotoluene solution. After drying the film in vacuum for 30 min the stable frequency (F1; Fig. 9) of the coated crystal was measured. The total polymer mass (m0) coated on the crystal corresponded to the frequency difference of (F1 — F0). CO2 was gradually pumped into the vessel via steep changes in pressure (see abrupt steep changes in frequency curve in Fig. 9) and the frequency data (F2 i, i =1 to 4) were continually recorded. As the polymer was insoluble at pressures lower than 1700 psi at 60°C, this set of frequency data at elevated pressures were used to evaluate the swelling (or CO2 absorption) of film and CO2 diffusivity in the polymer film. After the frequency of the crystal became stable at the last steep change in pressure (P =1520 psi), the CO2 pressure was instantaneously raised to the required experimental pressure (2400 psi) by adding more CO2 into the vessel. The QCM frequency went through a minimum value (F3) and increased rapidly, indicating the removal of polymer film from the crystal surface. The minimum frequency is the point at which CO2 absorption (film swelling) and film dissolution are balanced. Therefore in the example presented here, the dissolution of polymer film in scCO2 consists of two steps: 1) absorption-dominating and 2) dissolution-dominating. After the polymer film dissolved in scCO2 at 2400 psi and 60°C for 10 min, the CO2 in the vessel was rapidly released and the QCM frequency (F5) was measured again in vacuum. The remaining polymer film was removed by dissolution in pure trifluorotoluene (TFT), and excess TFT was dried off in vacuum. The original fundamental frequency (F6=F0) of the crystal was recovered, indicating complete polymer removal. On the polymer dissolution graph, the line from F3 to F4 represents the dissolution curve that can be used to evaluate the kinetics. Wu and Grant[50] have evaluated the dissolution kinetics of poly(FOMA-r-THPMA) films under the conditions that will be required for static film-developing and stripping in the microelectronics processing. Studies are currently underway to evaluate the dissolution kinetics of polymer films in a flowing scCO2 environment.
Fig. 8 Adsorption of scCO2 at 40°C on Ag-polished and Ag-rough QCM crystals. The inset on the upper right corner demonstrates how the adsorbed mass may alter the surface morphology.
ADDITIONAL QUARTZ CRYSTAL MICROBALANCE APPLICATIONS IN SUPERCRITICAL CO2
Absorption of Supercritical CO2 and Other Analytes in Solid Films
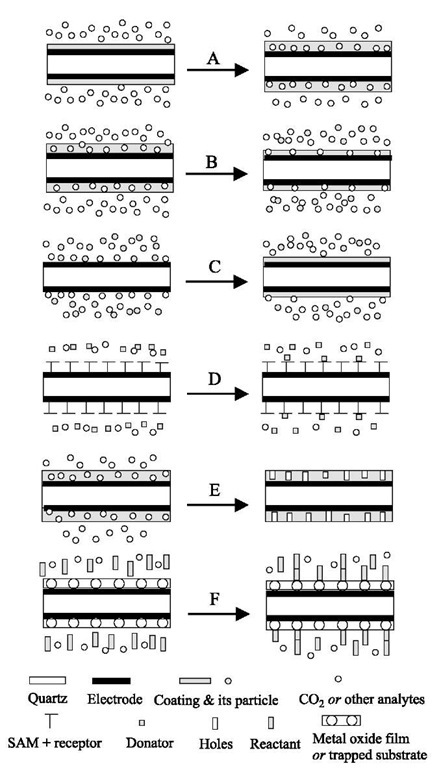
The absorption and solubility of scCO2 or other analytes in solid films are important properties that can be used to understand many scCO2-based processes and chemistries. The swelling of polymer films in scCO2 is a typical example of absorption processes. The QCM determination of absorption is realized by modification of the QCM surface with a coating that can interact with a desired analyte in a way that the mass and thickness of the coating are increased (Fig. 10A). To date, researchers[51-53] have successfully used the QCM technique to investigate the absorption of scCO2 in polymers at elevated pressures with high performance. There are a limited number of QCM studies on the absorption of an analyte other than CO2 into a solid film, where both the analyte and the solid film are contained in a scCO2 environment.
Interestingly, based on the QCM, some dual techniques have been developed to measure other absorption-relevant physical properties. Hattori et al.[54,55] combined vapor-liquid partition chromatography with a piezoelectric QCM to measure vapor-liquid equilibrium (VLE) ratios of benzene and toluene at an infinite dilution state in a scCO2-poly(vinyl acetate) system. The QCM indicated the solubility of organic solutes in the polymer phase while the chromatography analyzed the concentrations of solutes in the scCO2 phase. Good VLE data were obtained with this dual technique. Nakamura et al.[56] reported the determination of adsorption isotherms of scCO2 on several proteins and polysaccharides by a combination of two different microbalances, gravimetric and piezoelectric. The QCM was used to measure the absorption of CO2 into the proteins and polysaccharides; the absolute adsorption could be accurately calculated from the gravimetric data by subtracting the absorption contribution.
Fig. 9 A typical experimental QCM determination for the dissolution of poly(FOMA-r-THPMA) film in scCO2 at 2400 psi and 60°C under static conditions. Detailed explanation in the text.
Precision Cleaning and Dissolution Using Supercritical CO2
The QCM determination in cleaning and dissolution processes typically involves the partial or complete removal of a film or coating from the QCM surface by a liquid, gas, or a fluid in the supercritical state (Fig. 10B). Precision cleaning and precision decontamination can be defined as processes that result in a surface contaminant level of less than 10 mg/cm2.[57] However, most precision cleaning processes require a contaminant level of less than 1 mg/cm2.[58] A majority of recently developed scCO2-based precision cleaning processes are found in the microelectronic industry. Some typical examples are 1) the decontamination of micromechanical devices;[59] 2) the initial removal of impurities such as organic soils and fingerprints from silicon wafers; 3) the stripping of resists from metallized silicon surfaces without damaging thin metallization patterns;[60] and 4) the direct development of resist films.[10,61] More recently, the scCO2-drying of organic or aqueous rinse liquids from micro-electromechanical systems (MEMS) or other silicon structures were proposed to prevent the collapse of the fine features on the surfaces.[62,63] The QCM is capable of monitoring the mass changes in all these CO2-based precision-cleaning processes at the nanogram scale.
Solubility of Chemicals in Supercritical CO2
A thermodynamic property that is essential to any application of supercritical fluids is chemical solubility. The application of the QCM in the determination of solubility (Fig. 10B) is, in principle, very close to the approach used for cleaning and dissolution studies. A critical difference is the amount of time required for the attainment of phase equilibrium. The QCM method is ideally suited for solutes that exhibit low solubility, or for measurements conducted at low scCO2 densities, a condition in which many solutes have low solubility. This is because the QCM theory (Eq. 2) is considered accurate provided the mass of a coating does not exceed 2% of the crystal mass.[23] Guigard et al.[42] have applied the QCM technique to determine the solubilities of two metal chelates, bis(acetylacetonato) copper and bis(then-oyltrifluoro-acetonato) copper, in scCO2 and described the methodology in detail. They found that the solubilities measured by the QCM were in excellent agreement with the four sets of literature values obtained using other techniques, with a standard deviation of 25%.[42] This level of discrepancy is actually encouraging because of the extreme low solubility values on the order of 10-5 mole fraction. Besides metal chelates, the QCM method can be extended to determine the solubilities of natural products (e.g., herbal medicines), photoresists, polymers, fats, biomaterials, and many other chemicals of interest for CO2-based processes. This is especially true in cases in which the traditional detection methodologies (e.g., spectroscopic techniques) are limited due to either their low sensitivity or the complexity of the associated apparatuses under extreme pressure conditions.
Molecular Recognition in Supercritical CO2
Molecular recognition is of interest for biological processes because most biomolecules show selectivity and specificity when interacting with other species. This particular microweighing methodology involves either directly coating host molecules (insoluble in scCO2) on the surface of a QCM crystal (Fig. 10A) or modifying the surface with a self-assembled monolayer (SAM) (Fig. 10D). The SAM can be formed using thiols or other chain-like molecules that have two active groups at the two ends of the chain. The -SH group of the thiol or one end of the chain-like molecule is attached to the QCM surface, while the -OH group or the other end is grafted with the receptor molecule. When guest or donator molecules are supplied into the vessel together with CO2, they are bonded to the host or receptor molecules via molecular interactions, resulting in a mass increase of the coating. Naito et al.[64,65] successfully demonstrated two examples of molecular recognitions in scCO2 using the QCM technique. In the first system, ethyl acetate and ethanol were selectively bound to anthracene-bis(res-orcinol)tetraol that was coated on the crystal surfaces. In the second situation, nucleobases such as 9-ethyladenine and 2-pyrrolidone were recognized at different extents by thymine that was immobilized on the thiol-modified QCM surfaces. The equilibrium constants and the associated kinetics of molecular binding were easily obtained by the use of the QCM technique.
Evaluation of Deposition in Supercritical CO2
Chemicals either previously dissolved in the scCO2 phase or produced via reactions of other CO2-soluble chemicals may deposit onto surfaces, due to solubility variations with temperature and pressure or the insoluble characteristics. The dynamic mass of deposited surface films can be detected in situ using the QCM method (Fig. 10C). For example, the QCM technique can be directly used to evaluate the deposition of metals (e.g., copper and nickel) on silicon wafers.[66] The metals were generated via the hydrogen reduction of organometallic compounds that are dissolved in a scCO2 carrier. This deposition process was named as chemical fluid deposition (CFD), and the deposition of metals on pattern features less than 100 nm wide has been demonstrated using such CFD technique.[66]
Polymer Foaming in Supercritical CO2
In recent years, scCO2-based polymer foaming has been of particular importance in the production of low dielectric films in electronics manufacturing and tissue engineering scaffolds for biological purposes.[67] Upon removal of the scCO2 phase, the absorbed CO2 is released from swollen polymer networks, resulting in free-standing porous foams. The foams have cell diameters of less than 1 mm and the process accomplishes density reductions of 97% relative to the parent polymers.[68] Quartz crystal microbalance can act as a useful tool for detecting the dynamic mass changes associated with such foaming processes (Fig. 10E). The dynamic mass changes can be correlated with the structure and morphology of the foams by evaluation using microscopic techniques, such as SEM and AFM. The combination of the QCM microweighing with the microscopic techniques can provide a comprehensive understanding of the foaming processes.
Fig. 10 Schematic representations of some typical scCO2-based processes or chemistries that can be investigated using the QCM. (A) Absorption or molecular recognition; (B) cleaning, developing, stripping, drying, dissolution, or solubility in scCO2; (C) deposition; (D) molecular recognition; (E) foaming; (F) surface reaction, or polymerization.
Surface Reactions in Supercritical CO2
Reactions or polymerizations on metal oxide surfaces or other surfaces immobilized with active groups can be followed by detecting the mass increase on the surfaces with the QCM technique (Fig. 10F). When treated with acids, the metal oxide surfaces provide OH active groups to promote immobilization. The silylation of native oxide silicon surfaces in the preparation of coating for hydro-phobic photoresists[69] is a typical example of surface reactions in scCO2. Supercritical CO2 has been utilized as a carrier of silane reagents, thereby reducing the consumption of chemicals and improving the rate of surface coverage.
CONCLUSION
Two techniques, MSB and QCM, have been developed primarily as microweighing tools for wide use under extreme conditions. In the literature, the MSB has been utilized mainly for the determination of adsorption/ absorption and diffusivity of scCO2 in bulk polymers under static conditions. When compared to the MSB technique, the QCM has found a wider range of applications for many scCO2-based static or dynamic processes both in the bulk phase and at the surface film. This is primarily because of the intrinsic attributes of the QCM approach, such as the nanogram-level mass sensitivity, millisecond-level time resolution, miniature and simple construction, and low cost. The QCM can be successfully developed into a submicroweighing tool in scCO2 by the careful examination of the QCM theory in high-pressure fluids. Although the QCM has applications in various CO2-based processes and chemistries, such as dissolution, solubility, adsorption/absorption, and molecular recognition, the reported efforts and application areas in the literature are limited to date. It is evident from the progress reviewed in this topic that the QCM demonstrates great potential for use in scCO2.
Because scCO2 is a ”green” solvent, it is expected that the use of this solvent in nanoscale processing will increase significantly in the next few decades. The complete scope of the QCM in actual nanoscale processing has not been realized either. Hence the QCM will serve as an excellent analytical tool for developmental studies on emerging nanotechnologies and nanomaterials.