APPLICATIONS
Nanomaterials and nanotechnology are currently receiving major attention and publicity as a future market. While certain ceramic nanopowders have reached commercial status, nano metals are still in the research stage and sales average about 1 kg per order. Prices are hundreds of dollars per kilogram, and while substantial reductions in cost are anticipated, nanopowders will always be more expensive than conventional size powders. In addition, they bear higher shipping and handling costs. Therefore the focus of nanopowder suppliers is on applications, in which there is high value added by the nanosize particles. Target markets include energetics, microelectronics, metallurgical coatings, biotechnology, and niche powder metallurgy applications.
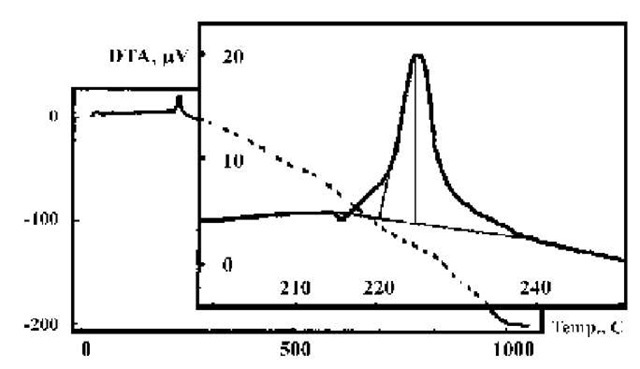
Fig. 7 DTA of EEW silver particles.
Energetics
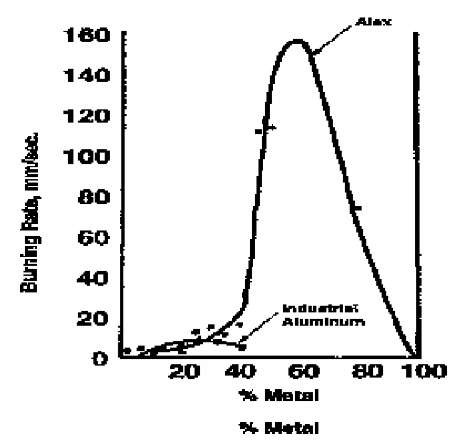
Aluminum is a highly energetic combustion fuel, particularly on a volumetric basis. For more than a century, it has been used as an additive in energetic compositions such as thermite, in explosives and pyrotechnics, and more recently, in rocket propellants. Because of its high surface area, nano aluminum provides a number of advantages over conventional aluminum powder, particularly with respect to burning rate. Fig. 8 shows that substituting Alex® for a conventional micron size 20 mm) aluminum in a mixture with ammonium perchlorate can increase the burning rate about 20 times.[8] Nano aluminum also ignites more rapidly. When combusted in an air shock tube, Alex® has an ignition delay of only 3 msec compared to 600 msec for 3-mm diameter aluminum powder.
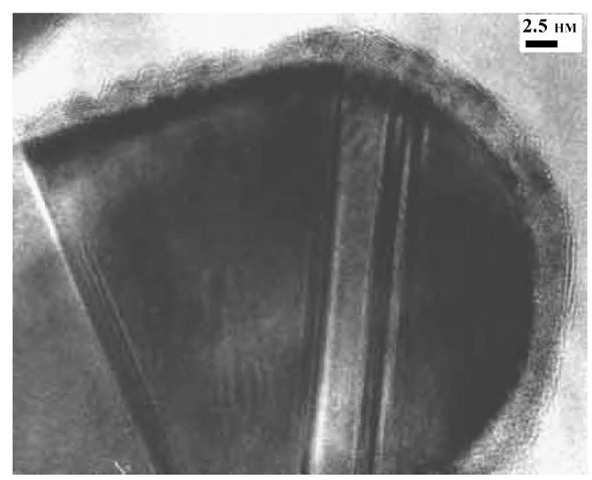
Fig. 6 Crystallographic defects in EEW nickel.
Fig. 8 Burning rate of aluminum/ammonium perchlorate powder mixes.
Rocket Propellants
Rapid burning increases the thrust and speed of a rocket engine, and higher thrust is desired in many advanced missile systems. Several researchers[8-10] have noted a doubling of burning rate when Alex® is substituted for micron size aluminum in conventional solid rocket propellants such as Al/ammonium perchlorate/hydroxy-terminated isobutylene binder (Al/AP/HTPB). The rapid burning is attributed to the smaller particle size and (much) larger surface area. A model developed for aluminum particle combustion in a rocket engine predicts that nano aluminum would burn very rapidly. This model[11] describes the life of the burning particle as proportional to the square of the particle diameter. Experimental data shows that a 5-mm aluminum particle survives for about 4 msec in a rocket engine. Extrapolation of the d2 model down to a 100-nm diameter predicts that the particle would be consumed in about 600 nsec, about 4 orders of magnitude shorter than the micron-size particle. High-speed photography of a burning propellant surface confirms that a nano aluminum particle is completely consumed at the surface of the burning grain rather than being propelled into the burning flow stream as in the case of the micron size aluminum. A faster burning grain is also more efficient because combustion is complete within the engine rather than in the exhaust stream of the rocket. The hybrid rocket engine would also benefit from nano aluminum as a fuel ingredient. The classic hybrid uses liquid oxygen with a rubber base binder (HTPB) grain that contains either no oxidizer, or just enough to react with the HTPB so as to be a gas generator. Pyrolysis of the rubber creates low-molecular-weight organic molecules that are forced into the engine and then react with liquid oxygen. If aluminum is added to a solid fuel such as HTPB, there is a theoretical increase in rocket performance, but unfortunately micron size aluminum does not burn effectively in such a hybrid. However, Chiavarinni et al.[12] found that adding 10 wt.% Alex® to an HTPB slab increased the regression rate by 70% and also resulted in smoother burning compared to the pure HTPB slab.
Aluminum, if gelled into kerosene, increases the volumetric energy density of the liquid rocket fuel. Unfortunately, micron size aluminum does not burn efficiently when immersed in kerosene; however, nano aluminum additive is completely combusted.[13] The higher temperatures created by combustion of nano aluminum also accelerates the combustion of the kerosene.
Explosives
Reshetov et al.[14] were the first to notice that nano aluminum influences the detonation velocity of high explosives. When less than 30 wt.%, nano aluminum is added to hexamethyl-3-nitroamine (HMX) (Fig. 9), detonation velocity (VoD) decreased from 5400 to 4700 m/sec, about equivalent to that of explosives when micron size aluminum is added to HMX. However, beyond 30 wt.%, there is a rapid rise in VoD to 7000 m/sec. Even where the VoD is not affected, the force of the detonation is enhanced by the rapid reaction of the nano aluminum with the gases generated behind the detonation wave.
Fig. 9 Detonation velocity as a function of aluminum in HMX explosive.
The affect of nano aluminum on enhanced detonation was demonstrated experimentally in the United States and Europe, and several organizations are studying Alex®’s use in explosives. When incorporated into ammonium dinitramide, Alex® was shown[15] to increase VoD from 4380 m/sec for a 97% dinitramide (balance Viton binder) to 5070 m/sec (73:24:3 ADN/Alex®/Viton). Identical loading of conventional aluminum had a detrimental effect on VoD. Similarly, detonation tube experiments comparing type 40XD flake aluminum and Alex® in N2 gas dispersed lactose/Al/ammonium perchlorate compositions showed VoD enhancements for Alex® over the flake grade at four different Al concentrations.1-16-1 Most recently, a collaborative program between Australian (DMSO-Adelaide) and Canadian (DREV) laboratories has demonstrated a beneficial enhancement for both VoD and brisance for a number of trinitrotoluene (TNT)-based tritonal and H-6 derivatives containing Alex®.[17] VoD enhancements of 200-300 m/sec and improvements in brisance of up to 27% were observed in a number of tritonal charges when conventional aluminum grades were substituted for Alex®.
Gun Propellants
When Alex® is added to gun propellants, the burning rate was nearly doubled as compared to high caloric conventional double-base propellants.[18] Simultaneously, the pressure exponent of Vieille’s burning law decreases from more than 0.8 for double base propellant to 0.66, resulting in more stable burning. Such aluminized gun propellants would be useful as a burning rate accelerator, as an igniter in high-pressure rocket propulsion, and as a booster.
Miscellaneous Pyrotechnics
Metal/oxide pyrotechnic heat sources (thermite) are enhanced with faster burning nanosize aluminum. Mixtures of nano size aluminum and nano MoO3 powder results in a very fast burning material. The reaction rate can be altered to develop energy release rates spanning the range between conventional explosives and conventional thermites. Such mixtures have application in advanced munitions, pyrotechnics, detonators, and primers.
Self-Heating Synthesis (SHS)
Refractory compounds or alloys may be synthesized by direct reaction using the heat generated by metal/metal reaction or as in thermite, where a metal reduces an inorganic compound. Table 2[19] shows several reactions in which EEW powders were used to form intermetallic alloys. At 200°C, a pellet of EEW copper and micron zinc powder can react within a second producing light and forming brass. Pressed pellets of Alex® and amorphous boron, when heated to 500°C and ignited by a hot wire, immediately forms aluminum diboride, while ordinary aluminum and boron would have to be heated above 1000°C for several hours to form the diboride. Nickel aluminide and alloys of aluminum with tungsten, iron, nickel, or molybdenum were produced at much lower temperatures and at shorter times than could be achieved by reacting micron size powders.
Electrically Conductive Inks and Pastes
The goal of shrinking circuits and increasing functionality has resulted in a continuing search for new and improved processes in electronic packaging. Metal powders such as copper, gold, nickel, tin, and solder are formed into pastes and used for electronic interconnects. Copper pastes are used in the production of hybrid multi-chip module (MCM) circuits. The pastes are printed on ceramics such as aluminum oxide and more recently aluminum nitride to produce highly dense, thick film circuits. Nanosize powders provide a flatter surface topology and more precise edge definition and line spacing that would be attainable as compared to conventionally sized powders. Precise patterning ensures lower cross-talk between adjacent conductor lines. While such better edge definition is not attainable in silk screening patterns because of resolution limits of the process, newer patterning methods such as photo-patterned thick film processes could benefit from the smaller features of nanosize particles.
Table 2 Alloying reactions in EEW pressed pellets
|
Metal 1 |
Metal 2 |
Reaction method |
Product/comments |
|
EEW Cu |
Coarse Zn—30% |
hot wire at 25C |
Brass verified by XRD |
|
EEW Al |
Amorphous B |
hot wire at 500C |
AlB2 produced |
|
EEW Al |
Coarse Ni |
hot wire at 25C |
Al-Ni alloy principal phase |
|
EEW Al |
Coarse Fe |
hot wire at 25C |
FeAl, FeAl3 and Fe2Al5 |
|
EEW Al |
EEW W (60 wt %) |
self-ignited at 300C |
WAl4 and WAl5 |
|
EEW Al |
Coarse Mo |
no reaction |
|
|
EEW Al |
EEW Mo |
self-ignited at 300C |
Al12Mo, Al5Mo and Al4Mo |
Metal-filled polymers also play an important role in microelectronics, including electrically conductive adhe-sives, polymers for shielding from radiofrequency radiation, and in magnetic polymeric layers. In most cases, high aspect ratio fibers and flakes are used because of the greater opportunity for conductor/conductor contact within the composite. Nano metal powders offer an advantage in increased electronic conductivity because of an increase in the number of point-to-point contacts. The authors developed silver-filled polyurethane and epoxy adhesives having an electronic conductivity of 1 x 10"5 and 2×10~6 O m, respectively, which are improvements over silver flake-filled polymers. The conductivity of the composite is enhanced by agglomerated particles that are particularly prevalent in EEW silver.
Nanostructures
Over the last few years, there have been extensive studies on nanostructures, with an expectation that they will form superplastic or ultrahigh strength, tough materials. Smaller grains result in greater strength, generally following the classic Hall-Petch relation, at least for grain sizes 50 nm and larger. Extrapolations forecast 2-7 times higher hardness and 2-3 times the tensile strength as compared to parts produced from conventional powders. Furthermore, the boundaries formed in a nanocrystalline structure tend to have higher ductility. Thus in contrast to most methods of strengthening metals, nanostructures have the potential dual benefit of increasing strength while also maintaining or increasing ductility.
There is a dilemma in that smaller grain size of a nano powder based compact recrystallize and grow at lower temperature, countering efforts to form nanostructures. Consolidation methods, such as equal channel angular extrusion (ECAE), that do not rely on much heating can densify the compact to greater than 99% while minimizing grain growth.
The breadth of potential applications for nanostruc-tured metals and alloys is considerable. The higher tensile strength and fatigue strength, and even the enhanced ductility that have been reported in nanostructured metals can impact any application in which strength or strength-to-weight ratios are critical properties. Transportation, aerospace, sports products, implantable medical components, and chemical and food processing applications appear promising.
Low-Temperature Sintering
The onset of sintering occurs substantially lower in temperature with nanosize particles. Eifert et al.[20] achieved a decrease in the onset of sintering of tantalum from about 1800 to 900°C when the particle size is reduced from 2 mm to 50 nm. The onset of sintering of 40 nm iron powder is as low as 370 K, approximately 21% of the melting point as compared to — 900 K, or 50% of melting for 2-mm-size iron powder.[21,22] The challenge of the nanoparticulate approach is that the pores are readily formed in nano sinters at low temperatures, and they tend to slow full densification unless there is some strain induced in the porous compact to prevent the stabilization of larger pores.
MISCELLANEOUS APPLICATIONS
Wear-resistant and microelectronic coatings can be formed from slurries of nanopowders. For example, the authors bonded a pattern of copper particles to glass by laser irradiation of a dried nanopowder paste deposited by silkscreen. The laser melted the individual particles to form circular disks approximately 1 mm in diameter in a line pattern with resolution of approximately 5 mm. The particles then provided a seed layer for the electroless deposition of additional copper to form a circuit pattern.
Selective laser sintering of metal powders is used for the computerized 3-D design and production of rapid tooling. Nanopowders are likely to be superior to conventional powders in that they sinter more readily and produce parts with tighter tolerance because of their smaller particle size.
Nanosize nickel-titanium alloys are being evaluated as source materials for producing memory alloy components.
Sintered metal disks are used in industrial filtration because of their capability to operate at elevated temperature and in corrosive environments. Disks produced from nanopowders would result in smaller pore size and would be more effective in filtering submicron particles.
A new class of heat transfer fluids is being developed where nanocrystalline particles are being suspended in liquids such as water or oil.[23] Copper oxide (5 vol.%) suspended in water results in an improvement in thermal conductivity of almost 60% as compared to water without nanoparticles. Direct evaporation of copper nanoparticles into pump oil results in similar improvements in thermal conductivity compared to oxide-in-water systems, but more importantly, requires far smaller concentrations of dispersed nanocrystalline powder.
Gold and other spheres are being considered for use as carriers of pharmaceutical and therapeutic agents through the blood stream to target organs. Colloidal gold particles have also been exploited in several bioanalytical methods, including a proposed DNA detection method.[24]
Iron powder, if injected into underground water plumes, will destroy trace halogenenated solvents. ”Iron walls,” which are permeable reactive barriers injected into the plumes, containing zerovalent iron, intercepts halogen-saturated solvents causing their de-chlorination. For chlorinated ethenes (perchlorethylene (PCE) and trichloroethylene (TCE)), the products are mostly fully dechlorinated although some chlorinated alkanes yield partial dechlorination products that may still be a pollution problem. There have now been many feasibility studies, pilot tests, small- to medium-scale demonstration projects, and full-scale applications performed by numerous groups. The authors have found that nano-iron is far more effective in converting per-chlorethylene to dichlorethylene than micron size iron. Also, nano iron has potential for the conversion of trace As [ + 3] to As [ + 5] so that it could be filtered from drinking water.
Nanosize particles interact differently with the electromagnetic energy spectrum than do micron size particles. For instance, solid particles, with sizes substantially below < 1/20 of the wavelength of light are transparent so that the film can be strengthened by adding inorganic particles without affecting transparency.
Nanosize metallic silver is being considered as a bio-cide for water purification and in medical formulations.
Nanosize copper is used as an additive in lubricating oils and sold in Russia. Tarasov et al.[25] showed that adding 0.5% nano copper to lubricating oil reduces friction in rubbing surfaces, particularly when under high load as in heavy-duty engines, thereby extending their life. The lubricating mechanism is believed to be the deposition of nano copper particles onto the surfaces of a hot friction pair, producing a softer metal surface on the aggravated surface. Such mixtures may prove to be superior to existing lubricants such as those containing Teflon particles.
Nanosize metal particles also have potential as precursors for the synthesis of a wide variety of inorganic compounds such as oxides with complex stoichiometry and with unique sizes, shapes, and reactivities. Furthermore, they have potential as precursors in the direct synthesis of metal organic compounds.
SAFETY, HANDLING, AND SHIPPING CONSIDERATIONS
Nano-metal powders are regarded as hazardous materials, particularly with respect to shipping regulations. Department of Transportation and International Air Transport Authority (IATA) require the user to test and categorize such powders relative to the combustion hazard prior to shipment. Should testing under the protocol show the powders to be pyrophoric in air, then shipment on passenger aircraft is disallowed. United Parcel Service and other carriers will not handle pyrophoric materials. The powders may be passivated by oxidizing their surface as in the case of nano aluminum, coating with an organic as in the case of L-Alex®, or immersion in a compatible liquid such as a hydrocarbon.
Working safety is also an issue with nano metal powders. Caution must be exercised to minimize the danger of untoward ignition and burning. Efforts should be directed to minimize the possibility of static ignition, particularly when there is an oxidant mixed in with the powder. Such oxidants include metal oxides and other oxidizing salts and halogenated organic liquids and solids. For instance, magnesium or aluminum mixed with Teflon powder is a highly reactive pyrotechnic. Users should study Material Safety Data Sheets (MSDS) before using nano metals. High efficiency particulate air (HEPA)-type respiratory filters should be used in operations where there is an opportunity for encountering nanoparticulate dust.
An issue common to most forms of nano metal powders is packaging to assure purity. Glass ampoules are superior to packaging in plastic containers, although ampoules would still not be acceptable under Department of Transportation (DOT) or IATA rules if the powder is pyrophoric. In this case, the powders have to be submerged in liquid hydrocarbon and sealed to prevent the ingress of air to minimize contamination.
CONCLUSION
The burgeoning field of nanotechnology is forecast to have an economic impact as great as biotechnology or microelectronics. Nano powders including nano metals are likely to be key source materials in this new industry. The EEW process is one of several methods being commercialized for manufacturing nano metal powders. EEW powders are readily produced from any metal or alloy that can be produced in the form of fine metal wire including elemental metals such as aluminum and copper, refractory metals such as titanium, tantalum, and tungsten, and alloys such as stainless steel and nickel-titanium.
Applications for nano metals include energetics such as rocket propellants, explosives and pyrotechnics, in self-heating synthesis of inorganic compounds and alloys, in high-strength nano structured metals, electrically conducting inks for microcircuits and in advanced capacitors, in environmental remediation, as carriers for bioactive and medical therapeutics, and in wear-resistant coatings. Most of the sales of nano powders are for small quantities to researchers investigating new properties with potential applications not discernable at this writing, but which are likely to add to the potential growth of this subindustry.