Magnetic Nanoparticle Sensors and Actuators
The capability to actuate ferrofluids through magnetic fields and magneto-mechanical coupling makes them attractive forMEMS/NEMS applications,[61,96-99] as well as for energy conversion schemes.[95]
The essential principles of magnetic nanoparticle sensors and actuators are illustrated in Fig. 9. Single magnetic nanoparticles can be trapped in nanofabricated wells surrounded by a nanocoil. The nanocoil can then serve to sense a voltage when open-circuited, or a current when short-circuited, or, with imposed current, can drive nanoparticle rotation, much like in the operation of macroscopic generators or motors. Imposed flow over the nanowell would serve to drag the magnetic nanopar-ticle into rotation. Such rotation would result in a time-varying magnetic flux in the nanocoil, causing induced open-circuit voltage or short-circuit current. The induced voltage or current can be measured and used to determine the flow rate of the fluid, or can be used as a micropower source converting flow kinetic energy to electrical energy. The reverse operation with proper multiphase time-varying currents, through a set of such coils to cause a rotating magnetic field, would result in magnetic nanoparticle rotation and flow actuation. Both single wells and arrays are possible, allowing for sensing and actuation of flow over whole surfaces of a microelectromechanical or nanoelectromechanical device consisting of nanoscale electromagnets, coupled with wells to hold the magnetic nanoparticles.
Fig. 9 Proposed surface-immobilized magnetic nanoparticle-based flow sensors and actuators.
The fabrication procedure for micron-sized electromagnets and micron-sized gold wires has been demon-strated[100,101] and was successfully used to trap and manipulate collections of magnetic nanoparticles and atoms. At liquid nitrogen or helium temperatures in vacuum, the wires could carry currents of several amperes with a current density of ~ 108 A/cm2 and a power dissipation of ~ 10 kW/cm2, producing magnetic fields to 0.3 T.
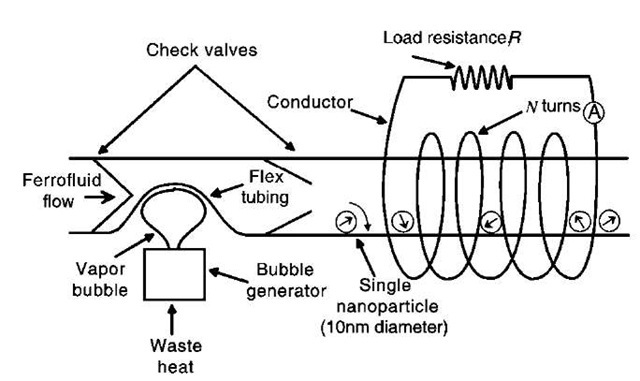
Representative present technology can fabricate gold coil windings that are about 3 mm thick and 10 mm wide, with center-to-center spacing of 20 mm, and can support a current density as high as 1011 A/m2.[100] A representative maximum current of 3 A produces a magnetic field of about 0.1 T. Fig. 10 extends the concepts in Fig. 9 and shows a proposed micropower supply method for possible space applications. Waste heat is converted to ferrofluid flow using a vapor bubble pump that control-lably causes magnetic particle rotation, which generates a time rate of change of magnetic flux through a nearby coil.[61] A 10 -nm-diameter magnetite nanoparticle with a magnetization of 0.56 T would have a total magnetic flux of about 4 X 10—17 Wb. If this nanoparticle rotated next to our representative coil at an angular speed of 1000 rps with all the magnetic flux of the nanoparticle linking the coil, the induced peak voltage would be about 3 X 10—13 V. The conductivity of gold is 4x 107 S/m so that the resistance of a gold wire of length 100 mm with a cross section of 10 X 3 mm2 would be about 0.08 O. The short circuit peak current would be the ratio of the voltage divided by the coil resistance, or approximately 4 pA. The maximum power that could be delivered by this single nanoparticle generator would be about 10— 24 W. If confined to a single plane, there could be as many as 1016 such single nanoparticle generators per square meter. If connected in series, the open circuit peak voltage could be as high as 3000 V/m2 of generator surface, whereas if connected in parallel, the short circuit peak load current could be as high as 40,000 A/m2 of generator surface, or in more practical units for nanoscale devices, about 0.04 mA/mm2 of generator surface area. The delivered time average power would then be on the order of 10— 8 W/m2.
Of course, practical packing constraints, small wafer sizes, smaller nanoparticle rotation rates, magnetic flux leakage (so that the nanocoils do not link all the magnetic flux), nanoparticle sticking, nanoparticle confinement, and other nonidealities and problems of controlling motions of nanoparticles would lower these idealized large values, but these ”back-of-the-envelope” preliminary calculations indicate the practical possibility of generating useful levels of micropower for selected applications, such as in space where plentiful waste heat can be used to cause ferrofluid flow to generate electricity, along the lines shown in Fig. 10.
Similar devices with submicron dimensions allow for single-particle manipulation and sensing. Trapping of nanoparticles would occur because of magnetophoretic forces,[102,103] analogous to dielectrophoretic trapping of particles,[104,105] where magnetic nanoparticles are attracted to the position of maximum magnetic field strength. A micromachined particle separator 2 X 3 mm in size was fabricated on a silicon wafer. Electromagnetic coils using 500 mA of d.c. current generated a ~0.03-T magnetic field to separate ~ 1-p.m magnetite particles from a water suspension at a velocity of ~ 1 mm/sec in a channel that was 90 mm deep and 100 mm wide.[103]
Fig. 10 Schematic diagram of a proposed micropower supply method that takes waste heat to cause a time-varying flow that controllably rotates ferrofluid nanoparticles so that there is a time-varying magnetic flux through a coil that induces a current through a load.
Microfluidics/nanofluidics
Ferrofluid devices can be a major component of a micro total analysis system (mTAS), also know as ”lab-on-a-chip.” The magnetic pressure generated in a ferrofluid is:
Ferrofluid plugs act as valves when placed near a stationary magnet and do not require any power. Hermetic sealing of a channel 250 mm wide, 150 mm deep, and 9.5 mm long has been achieved using a 400-G hydrocarbon-based ferrofluid with 42 cP viscosity and a 1200-G magnet, generating a magnetic field-induced change in pressure of pm « 13.5 Pa.[97] Ferrofluid microplugs have been able to sustain a pressure difference up to 1700 Pa.[99]
A number of micropumps use moving magnets to push ferrofluid microplugs that then displace nonmagnetic fluids.[96-99] A circular channel of ~ 1 cm diameter with 250 mm depth and 2 mm channel width containing a 125-G hydrocarbon-based ferrofluid pushing water had a flow rate of 45.8 pL/min with an 8-rpm motor rotating a 3500-G permanent magnet at speed 3.75 mm/sec, generating a pressure of 1200 Pa.[99]
An electromagnetic ferrofluid pipette was designed to accurately dispense liquid volumes smaller than 0.2 mL.[106] The pipette consisted of four electromagnets with a total of 160 turns around a 1.8-mm air gap holding the pipette tube. A small hydrocarbon-based ferrofluid plug of 600 G saturation magnetization and 1.43 Pa sec viscosity in the pipette would move with 0.6 A total current applied to the electromagnets, causing a ferrofluid pressure rise up to 770 Pa. The ferrofluid would move at a speed of 0.9 cm/sec in about 1.5 sec and would dispense 90 nL of water. The position of the ferrofluid plug was controlled to within 0.2 mm.
BIOMEDICAL APPLICATIONS
There are a number of methods in which magnetic nanoparticles can be used for biomedical applications:
a) Magnetic nanoparticles can bind to drugs, proteins, enzymes, antibodies, or organisms.
b) Magnetic nanoparticles can be directed to organs, tissues, or tumors using an external magnet for therapeutic effect.
c) Dissipation in alternating and rotating magnetic fields can cause heating of magnetic nanoparticles for use in hyperthermia.
Such chemotherapeutic applications require particles to be able to pass through organ and tissue capillary systems without threat of vessel embolism and be able to transport pharmacologically active materials with an effective release mechanism of the drug at the target site. All components for the drug carrier system must be nontoxic, biodegradable, and/or removable.[111]
As an example application, Fig. 11 shows a surface-immobilized nanoelectromechanical flow sensor or actuator that may operate for detection of biological molecules and organisms. Magnetic nanoparticles functionalized with a surfactant coating for specific binding to a biological molecule or organism would be placed in the nanowells. A biological liquid medium would then flow over the nanosensor array. If the biological molecule or organism of interest is present in the medium, then specific binding with the magnetic nanoparticles would result, thus hindering their rotation. This, in turn, would result in a measurable change in the induced current through the nanocoils. In this way, a surface-immobilized flow-through nanobiosensor may be developed.
Magnetorelaxometry is a technique used to measure the binding or immobilization of a magnetic nanoparticle through measurement of changes in magnetic relaxation time following the removal of an applied magnetic field. Small free nanoparticles have magnetization decay because of Brownian relaxation, with time constant tB of Eq. 4 increasing when additional molecules are bound to the nanoparticle because of the increased hydrodynamic volume Vh. The Neel relaxation time only depends on the magnetic material volume Vp and so is independent of nonmagnetic material binding to a magnetic nanopar-ticle. If the nanoparticle is bound to a solid phase and has restricted motion, the magnetization predominantly decays by Neel relaxation. By determining if magnetization relaxation is occurring through Brownian or Neel mechanisms, the binding conditions of nanoparticles to molecules or fixed material are determined.[112]
Fig. 11 Proposed surface-immobilized magnetic nanoparticle-based nanobiosensor.
Suitability for biotechnological and biomedical applications of magnetic nanoparticles and ferrofluids is being determined through fundamental studies of magnetic nanoparticle cell toxicity, biocompatibility, and transport for such applications as separations, magnetic resonance imaging, drug delivery, cytolysis (cell destruction), hy-perthermia, colloid extractants, cell sorting, and immuno-assays. A brief summary of current research follows.
Magnetocytolysis
Classical hyperthermia induces damage to cells and tissues, enhances radiation injury to tumor cells, and improves chemotherapeutic efficacy. A local thermal effect is desired within cancerous tissues while leaving normal tissues unaffected.[113,114] Heating between 41°C and 46°C generally reduces the viability of cancer cells and increases their sensitivity to radiation and chemotherapy. Temperatures up to 56°C (thermoablation) lead to cell death.[113-116] Magnetic materials for hyperthermia of biological tissues have been used since at least the late 1950s. Over a diameter range of 5-10 nm, the effective relaxation time of Eq. 5 in magnetite varies over two orders of magnitude. Ferrofluid subdomain particles 10 nm diameter) absorb more power at reasonable a.c. magnetic fields than by hysteresis heating of multi-domain particles 1 mm).[114-118]
Small amounts of magnetic fine particles in radio-frequency (rf) magnetic fields can easily rise in temperature to cause cell death. For example, for a magnetic field intensity of 6.5 kA/m at 300 kHz, Neel relaxation has peak dissipated power density of about 1010 W/m3 for magnetite nanoparticles of about 9 nm radius, decreasing to about 108 W/m3 at 7 nm radius and to 106 W/m3 at 12 nm radius. Hysteresis losses are about 106 W/m3 at 9 nm nanoparticle radius, rising to about 1010 W/m3 for nanoparticles larger than about 10 nm particle radius. Calculations and confirming measurements show that a temperature rise of about 16°C in about 30 min results from a 3-mm radius sphere of crushed magnetite embedded in a gel in a magnetic field of 17.6 kA/m at 300 kHz.[119]
To study the biological effects of a.c. magnetic field excited ferrofluids, both in vivo and in vitro studies have been done in cancer cell lines and spontaneously induced tumors in animal models.[118] The term magnetic fluid hyperthermia (MFH), which uses a.c. magnetic fields to heat target areas containing magnetic fluids, is suggested for this new cancer treatment method. It has been shown[120] that MFH is able to inactivate tumor cells in vitro to at least the same extent as water bath hyperthermia and that there is no cytotoxic effect of intracellularly administered dextran-ferrite magnetic particles.[121,122] An in vivo study[123] using transplants of a mammary carcinoma showed that high doses of MFH were able to induce tumor control in 44% of the cases up to 30 days after therapy.
Trials carried out on human and mouse macrophages (leukocytes, white blood cells) without a magnetic field and in a constant magnetic field did not observe any cytolysis or toxic effects.[124] Under an alternating magnetic field of 100 G at a frequency of 1 MHz, the temperature of a concentrated ferrofluid increases from 37°C to 80°C in about 2 min. Magnetocytolysis experiments were carried out using very low concentrations of magnetic nanoparticles, such that no increase in the temperature of the bulk solution was evidenced. After being subjected to alternating fields for 5 min, it was observed that 40-50% of the mouse and human macrophages are killed, but only 3-6 hr after application of the oscillating field. It was found that binding of the magnetic nanopar-ticles to the cell membrane is essential for cytolysis.
For iron oxide nanoparticles of 10 nm diameter deposited into tumors in mice and exposed to a 6500-A/m, 400-kHz magnetic field for 4 min, the local heating, called ”magnetic thermoablation,” raised the tumor temperature by ~ 62°C, causing therapeutic irreversible damage to the tumor.[125] Similar measurements in mice using water-based dextran-ferrite magnetic fluid in a 9300-A/m magnetic field at 0.88 MHz raised the tumor temperature by ~45°C, with 33% tumor regression and mouse life span increases of 150%.[126]
Drug Delivery
The use of biocompatible ferrofluids as a delivery system for therapeutic drugs is commonly called ”magnetic drug targeting.”[127-129] Mitoxantrone, a chemotherapeutic agent that inhibits DNA and RNA synthesis, was ionically bound to magnetic nanoparticles and was directed to cancerous tumor sites in rabbits using a 1.7-T magnet focused on the tumor. Complete tumor remissions with no adverse side effects were obtained using only 20% of the normal mitoxantrone dosage.[130] Similar success was achieved using ferrofluids labeled with iodine in a 0.6-T magnetic field.[131]
Magnetic targeting of anticancer drugs reversibly bound to magnetic fluids to locally advanced tumors can be achieved using magnetic fields directed to the tumor surfaces from outside the patient.[118,132] Phase I clinical trials using this approach in patients with advanced and unsuccessfully pretreated cancers or sarcomas showed successful magnetic drug targeting with epirubin[118] and epirubicin.[132] Based on magnetic resonance tomographic techniques, pharmacokinetics, and the histological detection of magnetite, it was shown that ferrofluids could be successfully directed by externally imposed magnetic fields to the tumors in about one half of the patients.
Superparamagnetic nanoparticles were surface-modified with poly(ethylene gycol) (PEG) and folic acid, respectively, to improve their intracellular uptake and ability to target specific cells.[133] It was found that PEG modification of nanoparticles resulted in decreased uptake by macrophages, whereas folic acid modification did not result in any change in uptake by macrophages. However, both PEG and folic acid modification resulted in increased internalization of magnetic nanoparticles in breast cancer cells.
Separations
Separation techniques are important for purification of biological materials and for identification of organisms, cells, and genomic materials. Application of a magnetic field to a suspension of magnetic nanoparticles confined between planar sheets causes the nanoparticles to self-organize into a regular array of columns. The column spacing can be controlled from submicron to 100 mm by varying gap width, nanoparticle size, and concentration. Such a self-assembled array of magnetic particles was used to separate large DNA in a microchannel in about 30 min, in contrast to the usual pulsed field agarose gel electrophoresis method, which takes about 12-24 hr.[134] Because pore size control from about 1-100 mm can be obtained without the need for sophisticated microlithog-raphy, there are many separation applications envisioned for DNA, cells, proteins, organelles, and microparticles or nanoparticles.
Magnetic fluids have also been used as magnetically responsive extractants usable in treating aqueous media contaminated with organic compounds. In one study,[135] magnetite (Fe3O4) nanoparticles of ~ 7.5 nm nominal diameter coated with a ~ 9-nm bifunctional polymer layer composed of a hydrophilic poly(ethylene oxide) (PEO) outer region and a hydrophobic poly(propylene oxide) (PPO) inner region were prepared. With the advantages of very high surface area and decreased diffusion lengths, these magnetic fluids were shown to have high capacity for organic solutes, similar to that of analogous PEO-PPO-PEO block copolymer micelles. Efficient magnetic nanoparticle recovery using high-gradient magnetic field separation (HGMS) has been shown and incorporated into a proposed water purification process.[135]
Magnetic cell sorting schemes based on quadrupole fields[136] and in microfabricated magnetic arrays[137] have been developed. The objective was to develop economical, high-throughput, high-purity, and high-recovery alternatives for separation of heterogeneous cell populations based on expression of particular surface markers.
An efficient magnetic vector/effector synthesis procedure was developed to target membrane receptors in target cells for subsequent separation.[118] This system was used to target, separate, and count damaged red blood cells. Applications of this technology include: 1) quality testing of human blood during storage in vitro;[138] 2) studies of the evolution of erythrocyte populations during parasitic pathologies such as malaria[139] and Alzheimer’s disease;1-140-1 and 3) detection of membrane modifications involved in complex biological processes such as apoptosis (programmed cell death).
Immunoassays
Immunoassays are widely used in biology and medicine for the determination of the composition of complex samples such as blood by labeling one of the reaction components with radioisotopes, enzymes, or fluorescent dyes. Magnetic nanoparticle labels offer advantages as they are inexpensive to produce, are stable, and can be safely and easily stored, handled, and disposed of without problems of radioactivity or environmental contamination.1-141-1
Antibodies were coupled with magnetic nanoparticles and reacted with their solid-phase adsorbed antigen. The samples were first placed in a magnetic field of 1500 A/m and then the remanent magnetization in the absence of magnetic field was measured in a superconducting quantum interference device (SQUID) magnetometer. This measurement technique investigated the binding of monoclonal antibodies (MABs) to collagen type III using Dextran-coated iron oxide nanoparticles with 13 nm mean diameter. Then polystyrene tubes used as sample containers were incubated with the antigen collagen type III so that the antigen was adsorbed onto the tube wall. The measured magnetic moment after switching off the magnetic field is entirely caused by the remanent magnetization of nanoparticles with hindered mobility because of their binding to the antigen fixed at the tube wall. Unbound nanoparticles relax by Brownian relaxation and have no net remanent magnetization. For this method to work, the Neel relaxation time must be longer than the measurement period, typically about 100 sec, which requires larger size magnetic particles on the order of >20 nm diameter for magnetite particles.[141,142]
Magnetic Resonance Imaging
Magnetic materials are highly opaque to x-rays and, with 8-10% volume concentration in ferrofluid, offer comparable image quality to conventional barium sulfate.[2,143]
Magnetic nanoparticles, conjugated to various MABs, peptides, or proteins, show potential applications for in vivo monitoring of brain intercellular communication and target-oriented magnetic resonance imaging in animal and human brains.[144] Dextran-coated superparamagnetic iron oxide is commercially available for MRI and has been used for localization and diagnosis of brain and cardiac blood obstructions and for liver lesions or tumors, where the nanoparticles tend to accumulate because of the difference in tissue fluidity and endocytotic processes (incorporation of substances into cells).
Approximately 7-nm-sized magnetite nanoparticles coated with polymeric starch were injected into a rat brain. The magnetic nanoparticles were well distributed within the dorsal hippocampus, demonstrating the feasibility of using magnetite nanoparticles for in vivo MRI of the rat brain.
Bacterial Threads of Nanomagnets
Magnetotactic bacterial threads can be used to direct the self-assembly of ordered magnetic microstructures.[145] Bacillus subtilis strain FJ7 is a mutant strain that can be cultured with intertwining filaments to form weblike structures. A thread was dipped into an aqueous colloidal suspension containing 10-nm-diameter magnetite nanoparticles between the cell strings to form threads of magnetic materials with an estimated nanopar-ticle density of 1018 particles/cm3 and a volume fraction of about 7%.
CONCLUSION
This article has described the integrated chemistry, fluid mechanics, and magnetic nanoscience and nanotechnolo-gy of magnetic fluids with summary reviews of ferrofluid synthesis: composition, colloidal stability, and preparation; ferrofluid magnetization: relaxation time constants, magnetization characteristic, magnetocaloric effect, and magneto-optical effects; ferrohydrodynamics: Bernoulli’s law of pressure and flow, instabilities, and rotating magnetic field torque-driven phenomena; applications to microelectromechanical/nanoelectromechanical systems: magnetic nanoparticle sensors, actuators, and microfluidic devices; and biomedical applications: drug delivery, hyperthermia, immunoassays, magnetic resonance imaging, separations, and cell sorting. These topics span the range from the nanosize magnetic nanoparticles to the concerted action from 1022 to 1023 magnetic nanoparti-cles/m3 to cause macroscopic pressure and flow. Continuing research will integrate ferrofluid nanotechnology to MEMS/NEMS fabrication capability by modification and tailoring of magnetic nanoparticle suspension properties for specific new high-technology applications as sensors, actuators, and biomedical tools.