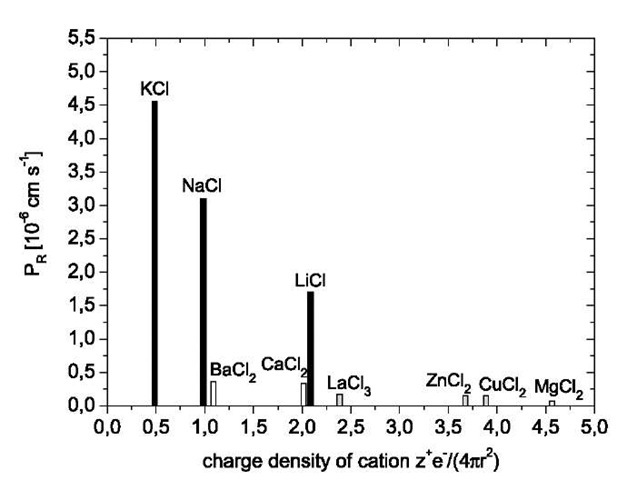
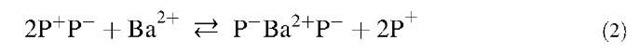Lebedev et al.[90] modeled the salt permeability of a fixed-charge multilayer membrane taking into account the charge number of the salt ions, the diffusion boundary layers flanking the membrane, and the different values of the ion diffusion coefficients in these layers and the membrane. The theoretical model was used in the Nernst-Planck equations together with the Donnan theory for the ionic equilibria at the membrane boundaries. Their calculations qualitatively reproduced the experimental results, but the theoretical permeabilities were considerably higher, if the experimental layer thickness was introduced.
The model of Donnan exclusion/attraction is based on the assumption that some of the polymer-bound charges within the polyelectrolyte multilayer are not balanced by polymer repeat units of opposite charge, but by small, exchangeable counterions. The excess polymer-bound charges act as ion-exchanger sites and are responsible for the high transport selectivity. The extent of polymer charge compensation in polyelectrolyte multilayers is still a matter of debate. In some studies,[91,92] evidence for incomplete polymer charge compensation and presence of small ions within the multilayers was found, while in other studies,[11,12] especially on polydiallyldimethylammonium bromide (PDADMA)/PSS multilayers, the polymer charge compensation was found to be essentially complete, the charge stoichiometry being 1:1. In that case, no selectivity in ion transport can be found, as was demonstrated by Krasemann and Tieke[43] studying PDADMA/PSS multilayers. Riegler and Essler[93] suggested that polymer charge stoichiometry depends on the charge density of the polyelectrolytes. If the distance between the charges is less than the Bjerrum length, the strong binding of the counterions (Manning condensation) prevents their release during multilayer formation, and the ion exchanger sites are permanently included in the film. This applies to the PAH/PSS multilayers, while in PDADMA/PSS membranes, the charge density is low enough to cause a complete complexation.
Fig. 6 Plot of the permeation rates of various chloride salts against the charge density z+e-/4pr2 of the nonhydrated metal cations (z+: charge number of ion; r: radius of metal cation). Membrane: 60 layer pairs PVA/PVS. Electrolyte concentration of aqueous feed solution: 0.1 M.
However, Farhat and Schlenoff[46] pointed out that small ion exchanger sites can be forced into these membranes if salt is added to the external bathing solution. Under these conditions, equilibrium (1) is partially shifted to the left because some of the polymer ion pairs dissociate. The authors demonstrated that the ion exchanger sites created in this way have a strongly nonlinear effect on the transport of redox-active ferro- and ferricyanide ions across the membrane.[46] The authors also devel-oped[94] a theoretical base for their observations by introducing a model of ion hopping between discrete sites of polymer-bound exchanger sites. However, the model does not take into account that several kinds of permeating ions, especially multivalent ions, may also receive other than electrostatic forces within the membrane. As reported by Toutianoush and Tieke,[95] di- and multivalent Cu2+, La3+, Ba2+, and hexacyanoferrate(II) ions become permanently fixed in PVA/PVS membranes, either because of a complex formation with the polymer-bound charged sites, as in the case of copper and hexacyanoferrate ions, or to a replacement of polymer-bound charged sites by the permeating barium ions, as indicated in Eq. 2:
Salt Transport Under Nanofiltration and Reverse Osmosis Conditions
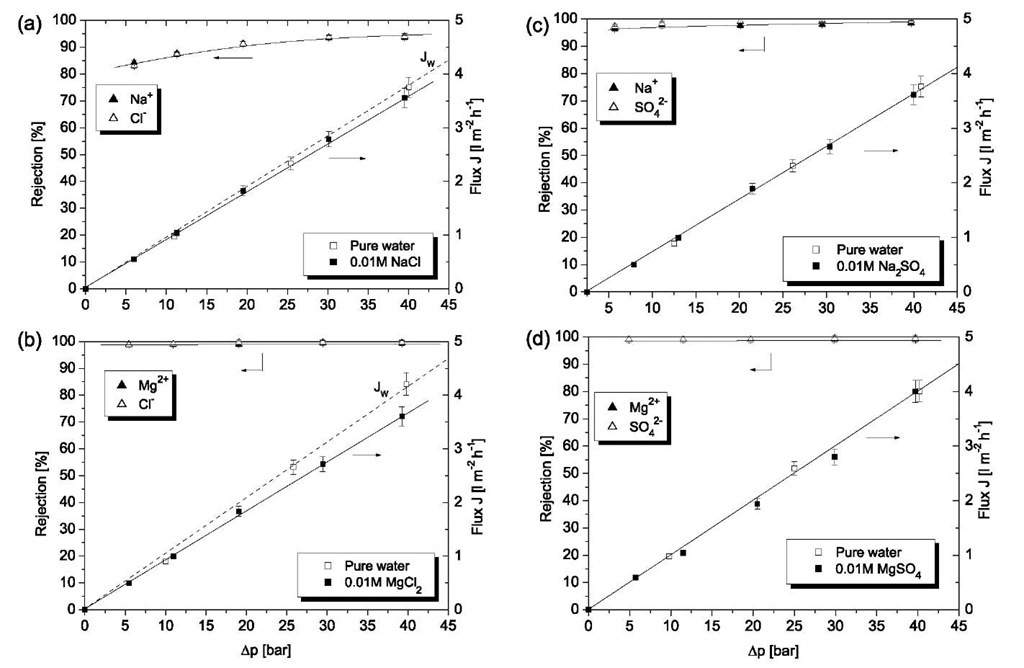
Very recent studies[49,50] were concerned with pressure-driven transport of aqueous inorganic salt solutions across polyelectrolyte multilayer membranes. Using either a dead-end[49] or a cross-flow apparatus,[50] ion permeation was investigated under nanofiltration (NF) (5-20 bar) and reverse osmosis conditions (more than 30 bar). In a first comprehensive study, Jin et al.[49] investigated the transport of aqueous electrolyte solutions containing NaCl, Na2SO4, MgCl2, and MgSO4 across multilayer membranes consisting of 60 bilayers PVA/PVS. For the two magnesium salts, a complete rejection was found independently from the concentration of the feed solution and the pressure applied (Fig. 7). For sodium chloride and sulfate, the rejections were 84% and 96% at 5 bar, and 93.5% and 98.5% at 40 bar, respectively. The ion rejection was comparable with those of commercial membranes, but the flux was lower because the less permeable PAN/ PET membrane was used as the support. Nevertheless, the study demonstrated that at low pressure, polyelectrolyte multilayers are suitable for NF applications such as water softening, while at high pressure, they can be used for water desalination. In a subsequent study, Stanton et al.[50] reported NF measurements across PAH/PSS membranes consisting of only five bilayers on porous alumina. Because of the ultrathin polyelectrolyte membrane, water flux was higher, but the ion rejection was lower than in the previous study. Again, the authors point out that the membranes are potentially attractive for NF applications such as water and salt purification.
Separation of Organic Compounds and Enantiomers
Besides small gas and solvent molecules or inorganic ions, polyelectrolyte multilayer membranes are also permeable for organic neutral molecules and ions provided they are small enough to pass the nanoporous structure of the membrane (Fig. 2). This allows the membrane to separate organic monoanions from dianions, for example, or naphthaline from the much larger pyrene or triphenylene, or phenol from hydroquinone.[96] If chiral polyelectrolytes are used for preparation of the membranes, even mixtures of enantiomeric compounds can be separated. In a recent study,[48] polyelectrolyte multilayers were prepared from polypeptides such as L- and D-poly(lysine) (PlD; PdD), l- and D-polyglutamic acid (PlGA; PdGA), and an optically active, quaternized polyvinylpyridine. As chiral probes, L- or D-ascorbic acid and 3,4-dihydroxyphenyl-L/ D-alanine were used. Selectivities of up to nearly 29% were found forD/L-ascorbic acid, if PdGA/PdL membranes were used, the flux was very high (Table 1). Multilayers made from two optically active polyelectrolytes were always more selective than multilayers containing only one optically active polyelectrolyte.
Separation of Proteins and Fouling Behavior
Polyelectrolyte multilayers were also deposited on substrates used for protein separation and filtration.[97] Celgard membranes were first treated with CO2 plasma, followed by grafting of polyacrylic acid to the surface. Then a bilayer of PDADMA/PAA was adsorbed. The bilayer-modified membrane showed a much lower tendency for protein adsorption and fouling than the unmodified membrane. The flux of an aqueous solution of human serum albumin (HSA) through the modified membrane was twice as high as the flux across the merely grafted membrane.
Muller et al.[98] studied the fouling behavior of CO2 plasma-treated polypropylene films modified with PEI/ PAA multilayers. For the modified films, the fouling was much lower than for the unmodified samples. Interaction of various charged model proteins such as HSA and lysozyme immunoglobulin G, and the polyelectrolyte multilayer were also investigated. Using infrared spec-troscopy, small amounts of adsorbed protein were found, if the top polyelectrolyte layer and the protein were equally charged, whereas enhanced adsorption occurred for electrostatic attraction between the protein and the polyelectrolyte layer.
Fig. 7 Plot of salt rejection and permeation flux J of 10- 2m aqueous solutions of sodium chloride (a), magnesium chloride (b), sodium sulfate (c), and magnesium sulfate (d) as a function of the operative pressure Dp. Experiments carried out at 20°C; feed solution stirred at 700 rpm. Jw indicates pure water flux. Membrane: 60 layer pairs PVA/PVS. Permeate solution was analyzed by measuring cation and anion concentration using high-performance liquid chromatography.
Table 1 Percent selectivities for different membranes and chiral probes
|
Percent |
Percent |
|
|
|
selectivity |
selectivity |
|
|
for AA |
for DOPA |
|
Membrane |
enantiomer |
enantiomer |
|
PlGA/PlL |
28.1 D/L |
14.1 L/D |
|
PdGA/PdL |
28.9 d/l |
13.8 D/L |
|
PdGA/PlL |
0.4 D/L |
0.3 D/L |
|
PlGA/PdL |
0.3 D/L |
0.1 D/L |
Graul and Schlenoff[99] also reported on the resistance of polyelectrolyte multilayers to irreversible protein adsorption. Fused silica capillaries were coated with poly-electrolyte multilayers and used for capillary zone elec-trophoretic separations. Basic proteins could be separated with good efficiency, and the columns were resistant to irreversible protein adsorption.
Other Work
In a number of studies, the transport behavior of poly-electrolyte multilayers was investigated on a more fundamental, less application-oriented base. In the following, some of the papers are briefly reviewed. von Klitzing and Mohwald[100] were the first to study proton and molecular transport through polyelectrolyte multilayers. Proton transport was investigated by using PAH/PSS multilayers on glass. A fluorescent, pH-sensitive dye was embedded in the multilayers at different distances from the glass surface and the pH dependence of the fluorescence emission was studied as a function of film thickness, surface charge, and ion concentration. The data showed that the films were permeable for protons. In subsequent work,[101] the time dependence of the fluorescence of a fluorescein-labeled PAH embedded at different depth in PAH/PSS multilayer films on glass substrates was studied. Adding rhodamine to the outer phase, a quenching of the fluorescence was observed as a result of energy transfer to the permeating rhodamine molecules. Paramagnetic quenching using a spin-labeled amine-N-oxide-based compound was also studied. Because this molecule was smaller than rhodamine, the diffusion coefficient was found to be at least 2 orders-of-magnitude larger.
Harris and Bruening[102] investigated the transport of hexacyanoferrate(III) and hexaammineruthenium(III) ions across PAH/PSS and PAH/PAA films on gold electrodes using electrochemical and ellipsometric measurements. The permeability of these ions was found to depend on solution pH, number of layer pairs in the film, presence or absence of a supporting electrolyte during film deposition, and the nature of the constituent polyelectrolytes. pH-dependent measurements of ion permeation were also carried out.
In other studies, polyelectrolyte multilayer membranes were subsequently cross-linked to reduce the permeability. Upon heating, it was possible to replace the polymer-bound ion pairs of PAH/PAA multilayers by amide link-ages.[103] Cross-linking of the multilayers at 130°C was found to reduce the permeability of hexacyanoferrate(III) ions to 1% of its original value. In another approach,[104] multilayer films were prepared from PAH and a maleate-based copolymer with carboxylic acid and mono-methylester groups. Heating in vacuum caused more than one-half of the amino groups to be converted into imide linkages with the substituent groups of the copolymer. After cross-linking, the films displayed selective permeation of methylorange against myoglobin (Mb).
Permeability studies were also reported from poly-electrolyte multilayer capsules. Transport of small molecules such as fluorescein, 6-carboxyfluorescein, pyrene, rhodamine 6 G, or ibuprofen through the multilayer wall was investigated, either from the outside to the interior of the capsules, or in the opposite direction.[105-110] The capsules were usually prepared from PAH and PSS. Capsules were found to be permeable in either direction, and the permeability could be controlled by a lipid coating of their surface,[111] by varying pH[108] or salt[109] concentration of the outside solution, or by annealing.[112] Effects of these parameters on the permeability were similar to flat multilayer membranes discussed above and thus are not further treated here.
CATALYST APPLICATIONS
The use of polyelectrolyte multilayers as catalytic surfaces is a rather new, but interesting topic, and comparatively few publications regarding this subject are available yet. Biocatalytic films may be obtained by incorporating enzymes into the films or binding them physically or chemically to the surface; inorganic catalytic films might be obtained by incorporation of inorganic nanoparticles in the films or binding catalytically active sites to the surface. A review on biocatalytic polyelectrolyte multilayers appeared recently.[113] In the next section, the research activities on biocatalytic multilayer assemblies are compiled; then, the multilayers containing inorganic catalysts are reviewed.
Biocatalytic Multilayer Assemblies
In an early study by Kong et al.,[51] negatively charged glucose oxidase (GOD) and bolaamphiphiles with bipyr-idine head groups were alternately built up on quartz slides and porous beads. For the assemblies, a high enzyme activity was reported. In a subsequent paper,[52] the same group prepared a bolaamphiphile-based multien-zyme reactor by using a 3-mercaptopropionic acid-modified gold electrode as support. The authors alternately adsorbed the cationic bolaamphiphile and anionic GOD or glucoamylase (GA). The multilayer assemblies catalyzed both the hydrolysis of maltose and the oxidation of glucose. The enzymatic activity increased with the number of GOD and GA layers, but the activity per layer decreased. The reason for this might be a limited diffusion of substrates in such condensed films.
In the following years, Kunitake et al.[53-57] prepared alternate assemblies of polyelectrolytes and enzymes and studied their catalytic activity. For example, PEI/GOD films were assembled on a quartz plate, and the reaction catalyzed by GOD was analyzed. GOD converts glucose and oxygen into gluconolactone and hydrogen peroxide, and H2O2 oxidizes an indicator dye in the presence of peroxidase (POD) in aqueous solution. Finally, the blue oxidation product of the dye is monitored by ultraviolet (UV) spectroscopy. The study[55] clearly demonstrated that the activity of the enzyme was not lost in the multilayer assembly. In another study,[56] a multienzyme reactor containing PEI/GOD and PEI/GA bilayers was constructed on an ultrafiltration membrane. For the study of enzymatic activity, an aqueous starch solution was employed. When flowing across the membrane, GA decomposed starch to glucose, and GOD converted glucose to gluconolactone, thereby producing hydrogen peroxide as the product. The H2O2 concentration was analyzed as described above. A high catalytic activity was found, and the efficiency of the glucose oxidation was observed to be affected by PEI/PSS intermediate layers, flow rate, and starch concentration. In another study,[57] it was found that premixing of GOD and PEI followed by alternate adsorption with PSS showed a significant increase in enzymatic activity.
Coating electrodes with enzyme/polyelectrolyte multilayers provides the basis of a biocatalytic device including an enzymatic bioreactor. Lvov et al.[58] prepared myoglo-bin (Mb) containing alternately assembled films at an electrode. Electrochemical reduction of the FeIII in Mb to FeII is accompanied by H2O2 formation from oxygen. H2O2 is able to oxidize FeIII in the Mb into the active oxidant form ferrylmyoglobin (MbFeIV), and is thereby reduced to water. In the paper, it is demonstrated that MbFeIV is able to epoxidize styrene at the multilayer surface. Calvo et al.[59] demonstrated that GOD, lactate oxidase, and soybean peroxidase can be electrically wired to an underlying electrode through PAH with a covalently attached osmium complex. This multilayer system was able to indirectly drive lactate oxidation by the electrode via the corresponding enzyme. Earlier, Hodak et al.[60] assembled GOD with a redoxactive poly(allylamine)ferrocene mediator on alkanethiol modified gold electrodes. GOD was found to be catalytically active in glucose oxidation, but only a small fraction of the active enzyme was electrically wired by the ferrocene polymer. Ma et al.[61] reported on electroactive Mb films grown layer-by-layer with PSS on pyrolytic graphite electrodes. Cyclic voltammetry showed the expected reversible peaks of the Mb FeIII/FeII redox couple. Oxygen and trichloroacetic acid could be catalyt-ically reduced by the Mb in the multilayer assemblies. He et al.[62] reported on multilayer devices consisting of PDADMA and the anionic bacteriorhodopsin (BR). The biological activity of BR in these assemblies could be confirmed by measuring the generation of a photocurrent.
Use of spherical particles as substrates for the enzyme-containing multilayer assemblies is advantageous because these particles offer a high surface area and a large amount of enzyme can be incorporated. Schuler and Caruso[63] reported on the construction of enzyme/ polyelectrolyte multilayer films on submicrometer-sized polystyrene (PS) spheres using the layer-by-layer approach: PEI and glucose oxidase (GOD) were alternately adsorbed. Up to three layer pairs were deposited. The enzymatic activity was measured by following the coupled enzymatic reaction of GOD and horseradish per-oxidase. The activity increased regularly with increasing number of GOD layers immobilized, indicating that the multilayer films were sufficiently permeable for substrate diffusion. In another study,[64] GOD, POD, or preformed enzyme-polyelectrolyte complexes were assembled in alternation with oppositely charged poly-electrolytes onto PS particles. Particles coated with the preformed enzyme-polyelectrolyte complexes displayed a significantly lower enzymatic activity (by up to 70%) than those fabricated by direct adsorption of the free enzyme. Furthermore, experiments were conducted with particles exhibiting both magnetic and catalytic functions. Magnetic support materials are widely used in biotechnology because they can easily be separated. For this purpose, 200 nm PS particles that were precoated with four layers of Fe3O4 nanoparticles and PDADMA, followed by two PAH/PSS additional polyelectrolyte layers and an outer GOD layer were prepared by using the layer-by-layer approach. Then, the particles were repeatedly used as catalysts following their rapid and easy separation with a magnet.[64] Santos et al.[65] constructed biocatalytic thin films by incorporating enzymes, specifically alkaline phosphatase (AP) and GOD into multilayers of PEI and PSS supported on a glass substrate. For both enzymes, the reactive films demonstrated increased activity with the successive number of deposited enzyme layers. In hybrid films consisting of alternating layers of AP and GOD, both enzymes retained their activities similar to those of their corresponding films of either enzyme alone.
Biocatalytic and magnetic particles prepared upon the layer-by-layer approach were also reported by Fang et al.[66] The authors started from 420-nm latex particles, onto which they alternately assembled silica or magnetite nanoparticles, GOD, and oppositely charged polyelec-trolytes. The inclusion of silica layers on latex yields a higher surface area resulting in greater GOD adsorption, thereby increasing the catalytic activity. The enzymatic activity was proportional to the core surface area and also to the number of GOD layers in the shell. The presence of magnetic nanoparticles allowed the self-stirring of the nanoreactor with a rotating magnetic field and enhanced the productivity.
In a very recent study,[67] enzyme activity of organo-phosphorus hydrolase (OPH) in polyelectrolyte-encased multilayer assemblies coated on glass beads was reported. The stability of the multilayer systems (e.g., against exposure to high salt concentration) could be strongly enhanced by anchoring monomers, such as 1.2-dihy-droxypropyl-4-vinyl-benzylether, to the outer PAA layer and subsequently polymerizing the systems by UV irradiation. The OPH activity was not affected. Preparation of high-activity, enzyme-containing multilayer films on planar substrates was reported by Jin et al.[68] Catalase microcrystals were first encapsulated by the alternate adsorption of PSS and PAH on their surface yielding extremely high enzyme loading in the polyelectrolyte multilayer capsules. Subsequently, layer-by-layer deposition of the polyelectrolyte-coated catalase microcrystals and oppositely charged polyelectrolytes was used to construct multilayer films on glass supports. The catalyze achievement of these high enzyme content films was up to 50 times higher than of those prepared by conventional layer-by-layer deposition of the enzymes.
Multilayer Assemblies Bearing Inorganic Catalysts
Polyelectrolyte multilayers are not only able to bind bioactive compounds, such as enzymes to produce highly efficient biocatalytic surfaces, but also inorganic compounds with high catalytic activity was reported. There are many studies on the incorporation of small inorganic particles in polyelectrolyte multilayer assemblies, but applications of these systems as catalysts are only scarcely demonstrated. Mecking and Thomann[69] reported on the adsorption of a catalytically active rhodium complex on anionic latex particles, which were previously coated with a PDADMA layer. The rhodium complex was bound to the PDADMA layer via electrostatic interactions of its sulfonated phosphine ligands. The structurally well-defined core-shell particles were able to catalyze hydroformylation reactions such as the conversion of methyl acrylate into methyl 2-formyl-propionate. Wang et al.[70] fabricated PAH/PAA multilayers by using the layer-by-layer approach and selectivity bound palladium catalysts for electroless nickel plating to the surface. Depending on whether the surface was cationic or anionic, either the negatively charged tet-rachloropalladate or the positively charged tetraamine palladium ion was bound to the surface. Upon immersion into an electroless nickel plating bath, the palladium-coated parts of the surface were selectively plated. Using inkjet printing, the facile patternability of the polyelectrolyte multilayers was demonstrated. Wang et al.[71] also prepared palladium nanoparticles within PAH/PAA multilayers as seeds for binding tetraamine palladium from aqueous solution to PAA carboxylic acid functionalities in the polymer film and subsequent reduction. Two-nanometer catalytic palladium particles were obtained, onto which a nickel shell of arbitrary thickness could be grown. Up to 14-nm particles were obtained. Liu et al.[72] described a similar route to the fabrication of polyelectrolyte multilayers containing catalytically active palladium nanoparticles. A 4-aminobenzoic acid-modified glassy carbon electrode was alternately coated with tetra-chloropalladate dianions and a quaternized poly(4-vinyl-pyridine) complexed with osmium. Three-dimensional palladium particle multilayers were subsequently formed upon electrochemical reduction of the tetrachloropaladate anions. The palladium nanoparticles in the film effectively catalyzed the reduction of dissolved oxygen and the oxidation of hydrazine compounds in aqueous solution. Antipov et al.[73] prepared PAH/PSS multilayer capsules containing silver ions. Subsequently, the silver ions were reduced via photoirradiation, or by chemical methods. The potential of the prepared silver-containing capsules to serve as catalysts was checked by the reduction of 4-nitrophenol into 4-aminophenol in the presence of sodium borohydride. The system under investigation was found to possess higher activity than the pure silver solution.
In some studies, multilayers of redoxactive compounds and polymers were assembled on electrodes to obtain electrocatalytic surfaces. Sun et al.[74] prepared layer-by-layer assemblies of polycations bearing an osmium complex and PSS or poly(aniline-co-N-(3-sulfopropyl)aniline) on gold electrodes. The chemically modified electrodes showed electrocatalytic response for the reduction of nitrite. Cheng and Cox[75] used the layer-by-layer approach to prepare nanocomposite multilayer films of a ruthenium metallodendrimer and a Dawson-type polyoxometalate. These multilayers were used as bifunctional electro-catalysts, able to catalyze both reductions and oxidation. Using cyclovoltammetric studies, the mediated reduction of iodate by the polyoxometallate (phosphotungstate) and the oxidation of arsenite by the ruthenium dendrimer were demonstrated. Photocatalytic porphyrin multilayer assemblies were reported by Araki et al.[76] Electrodes modified with these films were photocatalytically active toward the reduction of O2.
Finally, Sasaki et al. prepared multilayer films of PDADMA and titania nanosheet crystallites,[77] and of titania nanoparticles and PSS.[78] UV irradiation of both types of films did not cause the expected photocatalytic decomposition of pollutants or water, but instead, the entire organic polyelectrolyte inside the multilayers was decomposed, and a polymer-free inorganic film was obtained. The photocatalytic reaction was monitored via infrared and X-ray photon spectroscopy.
CONCLUSION
In the last decade, a variety of studies demonstrated that the alternate layer-by-layer assembly of cationic and an-ionic polyelectrolytes is a suitable method to prepare ul-trathin, dense separation membranes on solid porous or pore-free supports. The main advantages are the simple, yet elegant preparation method; the environmentally sound, purely water-based chemistry; the use of low-cost, easily available compounds; the potential to precisely control structure and thickness of the membrane in the nanometer range; and the wide variety of compounds that can be chosen for the construction of the membranes. Previous studies also demonstrated that polyelectrolyte multilayers represent multipurpose membranes, which are well suited for dehydration of organic solvents under pervaporation conditions, for separation of mono- from multivalent ions under dialysis conditions, for water softening and desalination under nanofiltration and reverse osmosis conditions, as antifouling coatings for protein separation. Although the thickness of the multilayer membranes is usually below 100 nm, the efficiency of the membranes is comparable to commercial membranes of macroscopic thickness applied in modules.
The nanoporous, physically cross-linked structure of the membrane favors the permeation of small, hydrophilic molecules and ions of low charge density. In general, the selective transport behavior and the possibility to control the transport by adjusting pH and ionic strength of the feed solution, or by a chemical modification of the membrane, are two of the most interesting properties of polyelectrolyte multilayers. Future studies will be concerned with further improvement of the efficiency of the membranes, either by modifying hydrophilicity or cross-linking density, or by incorporating permselective compounds such as calixarenes, cyclodextrines, zeolithes, or inorganic complex salts acting as molecular filters and ion sieves. Furthermore, studies will be concerned with a better theoretical understanding of the transport behavior.
For catalyst applications, the electrostatic buildup of polyelectrolyte multilayers with catalytic sites either embedded in the films or physically attached at the surface offers great advantage over covalent coupling and chem-isorption methods. As recently demonstrated, the adsorbed or embedded enzymes and inorganic nanoparticles retain, at least partially, their catalytic activity rendering the multilayer assemblies attractive as new bioactive and catalytic materials. Although this review may be incomplete, the examples presented indicate a high potential of the polyelectrolyte multilayers to serve as carriers for catalytic substances. The experiments indicate that a binding of the catalyst to the surface leads to higher efficiencies than the dispersion of the catalyst within the multilayer. Furthermore, the use of spherical particles or capsules as a substrate is superior to a binding of the catalysts to flat multilayers. The reason is the much larger surface area providing a higher catalytic activity. Future work will be concerned with more systematic studies on incorporation, mobility, and activity of catalytic compounds in polyelectrolyte multilayers. Further systems demonstrating the suitability of polyelectrolyte multilayers as bioreactors and chemical nanoreactors need to be developed.