INORGANIC INTERFACES TO SWNTS
While nanotubes have been treated with dichlorocar-bene and Birch reduction conditions (i.e., lithium in 1, 2-diaminoethane),[52] recent efforts have been involved with generating more controllable derivatization of SWNTs, both spatially and structurally. One of the ideas pursued by our group was to derivatize SWNTs with relatively bulky inorganic complexes, which not only yields a novel metal-based molecular coordination complex but also offers the possibility of tailorable solubility in a variety of solvents, through mechanisms such as charge-mediated stabilization along the length of the tube and ligand exchange, without completely disrupting the tube’s electronic structure. In addition, these generated adducts can be used in a number of different catalytic processes, including homogeneous catalysis, upon which the expensive metal-support assembly can be facilely recovered from solution. As another example of the utility of metal complexation, nanotubes, functionalized with a ruthenium complex, for example, have been utilized to create interconnects showing multiple T- and Y-junctions.
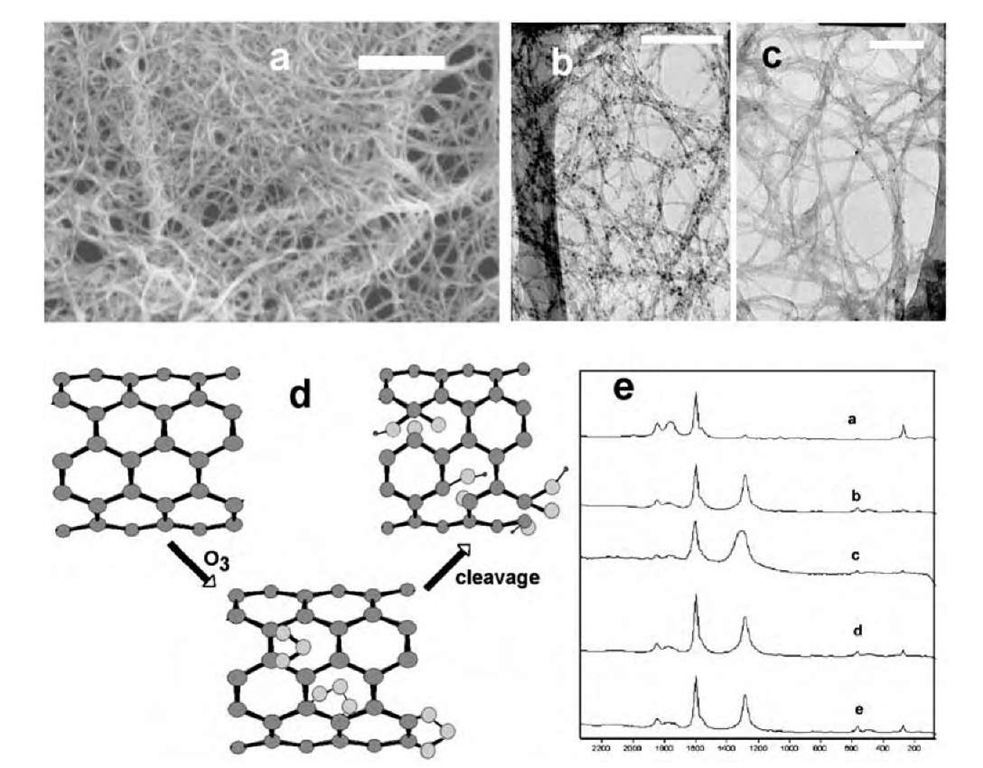
Fig. 5 (a) Scanning electron micrograph (SEM) of ozonized tubes. Scale bar is 1 mm. (b) Transmission electron micrograph (TEM) of as-prepared HiPco tubes. (c) TEM of ozone purified HipCo tubes. Scale bars in panels (b) and (c) are 130 and 140 nm, respectively. (d) Proposed reaction scheme for ozonation. The primary ozonide that is initially formed is cleaved to yield functional groups on the sidewalls. (e) Raman spectra show an increase of the D band upon ozonation, implying sidewall functionalization and considerable perturbation of the electronic structure.
Specifically, both oxidized nanotubes and raw, unfunc-tionalized SWNTs have been reacted with Vaska’s compound, trans-IrCl(CO)(PPh3)2, to form covalent nano-tube-metal complexes.1-54-1 Moreover, the complexes of oxidized SWNTs with Vaska’s compound are much more soluble in dimethylformamide (DMF) than raw tubes, a finding that should facilitate the chemical manipulation as well as photophysical analyses of these materials. In fact, the solubility of oxidized tubes varied with the concentration of Vaska’s complex added, suggesting that dissolution was chemically induced. The solubility of a typical adduct sample in DMF at room temperature was — 250 mg/L, about 1 order-of-magnitude improvement over that found for unmodified tubes.[55] Analogously, the SWNT adduct with Wilkinson’s complex[56] (Fig. 6) shows a relatively high degree of solubility in dimethyl-sulfoxide (DMSO), reproducibly >150 mg/L, and roughly 30% of that value in DMF and tetrahydrofuran (THF). Our results with oxidized nanotubes, but otherwise unmodified with any of these metal complexes, showed a solubility of – 25 to 35 mg/L in DMF and 4-10 mg/L in THF. Conceptually, the novelty of this derivatization is that the nanotube can be considered as a primary ligand, as a functional moiety like any other, with respect to the central metal atom, Ir. Results were confirmed by 31P nuclear magnetic resonance (NMR) spectroscopy, FT-IR spectroscopy, electron ionization mass spectroscopy, as well as data from transmission electron microscopy (TEM) and energy-dispersive X-ray analysis (EDX).
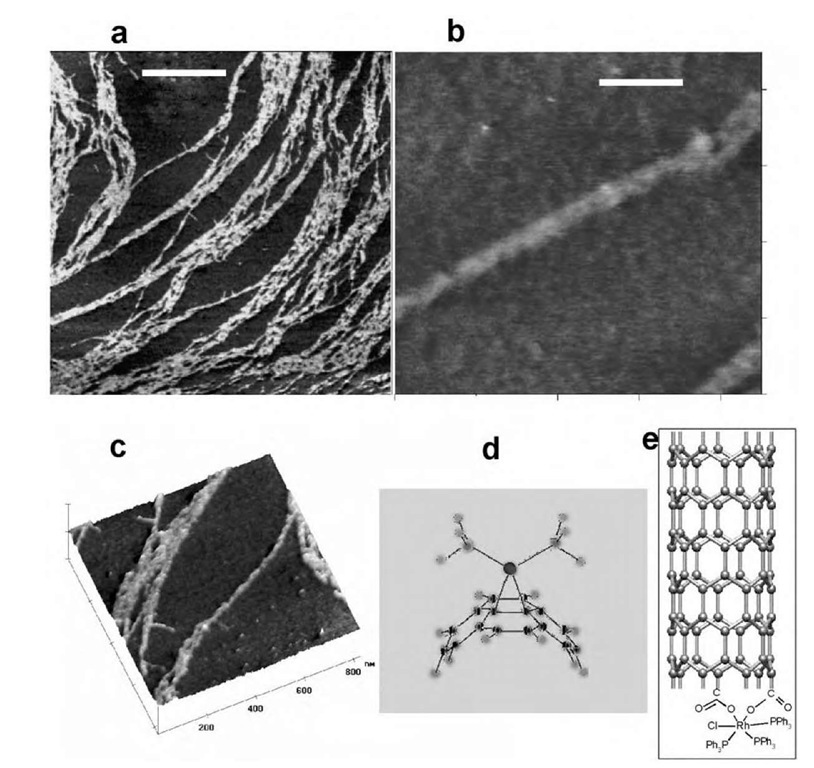
Fig. 6 Atomic force microscopy (AFM) height images of functionalized nanotube adducts. Scale bars are (a) 500 nm, (b) 100 nm, and (c) 200 nm. (a) A high density of tubes has been deposited from solution. Aggregates of tubes appear to be exfoliating into smaller bundles. (b) Image of a single bundle roughly 15 nm in diameter. (c) A 3-D view of exfoliating tubes. The bundles are relatively clean and free of nanoparticulate impurities. (d) Optimized geometry of M(PH3)2 coordinated in an z2 fashion to SWNT sidewalls. (e) Proposed structure of SWNT-Wilkinson’s adduct. Panel (d) is based on a schematic provided by Professor Antonio Sgamellotti (Universita di Perugia).
To summarize, Vaska’s compound complexes to raw nanotubes by Z2 coordination across the graphene double bonds. In effect, ligand binding is an associative process with the formation of a p complex and is expected to be similar to that observed[57,58] in (TCNE)IrBr(CO)(PPh3)2 or in the fullerene adduct.[59] This type of reaction, in which the ligand acts as a weak base toward the metal complex, has been referred to as a ”reductive addition” (with respect to the metal).[60] For the adduct formed with the oxidized SWNTs, coordination through the oxygen atoms seems a more likely possibility, in other words, through an oxidative addition to form a hexacoordinate structure. Oxidative additions to form 18e- ”coordina-tively saturated” compounds are well known for Vaska’s compound and other related d8 complexes and have been extensively studied.[61,62] Thus it is postulated that the oxidized nanotubes coordinate to the compound via oxidative addition to form an Ir(III) complex. These conclusions are in general agreement with theoretical predictions, based on a cluster model approach and a density functional theory study.[63-
The reactivity of SWNTs toward metal complexes is substantially different from that of fullerenes, although both possess a nonplanar sp2 configuration. Curvature effects and different degrees of local strain between the two structures likely account for the observed differences in behavior. Specifically, the presence of five-membered cyclopentadienyl-like rings in C60 substantially enhances the affinity of (6,6) bonds in coordinating metal complexes and allows for strong back donation in the resulting adducts, thereby promoting their stability. However, theoretical calculations on molecular fragments replicating SWNT surface curvature indicate that, unlike in the case of fullerenes, five-membered rings are not present to stabilize p* ligand orbitals and hence, backbonding interactions are much weaker in these systems.[63] This would rationalize the preference for oxidative addition, when that should become a possibility.
Single-wall nanotubes have also been used as substrates for the deposition of metals by electron-beam evaporation, resulting, in the case of Au, Al, Pb, and Fe, in the formation of discrete particles on nanotubes due to a weak interaction between metals and nanotubes.[64] Acid groups on treated SWNTs can act as nucleation sites for a well-dispersed deposition of Pt clusters.[65] With Ti, Ni, and Pd, quasi-continuous coatings are possible, resulting in the formation of nanotube-supported metal nanowire structures.1-66-1 Zinc oxide nanowires have been grown on the surfaces of MWNTs without the presence of catalyst through a thermal treatment.1-67-1
Our group has been involved with the generation of nanotube heterostructures as well, involving the creation of nanotube-nanocrystal adducts. Indeed, a novel strategy of altering the electronic properties of nanotubes is to chemically functionalize them with a moiety or structure whose intrinsic properties are electronically configurable. One such structure is the family of semiconductor nanocrystals,[68- such as CdS and CdSe, alternately known as quantum dots, which exhibit strongly size-dependent optical and electrical properties. The high luminescence yield of these materials1-69-1 as well as the potential of adjusting emission and absorption wavelengths by selecting for nanocrystal size make quantum dots attractive for constructing optoelectronic devices, for instance, with tailored properties. In fact, the electronic structure of semiconductor nanocrystallites exhibits distinctive quantum effects;1-70-1 the bandgap of these materials increases with decreasing particle size.
We have reported1-71-1 the synthesis and characterization of SWNTs covalently joined to CdSe and TiO2 semiconductor nanocrystals by short chain organic molecule linkers (Fig. 7). In each case, purified, oxidized nanotubes were reacted with nanocrystals, derivatized with either amine or acid terminal groups in a reaction mediated by a carbodiimide reagent. By judiciously varying the nature of the organic capping groups on the nanocrystal surface and the organic bifunctional linkers, we can modulate interactions between the nanotubes and the nanocrystals, with implications for self-assembly. Such composites are expected to be useful for applications as diverse as molecular electronics, photocatalysis, solar energy conversion, and as probes for scanning force microscopy.
TEM examination (Fig. 8) showed nanocrystals linked to the oxidized SWNTs, forming discrete nanocomposites. The nanocrystals tend to be concentrated at the open caps and the ''ends,'' where there is the largest concentration of carboxylic groups[72,73- and thus, the highest probability of amide bond formation.[74- A commonly observed structure was a cluster of nanoparticles, especially in the case of CdSe, localized at the outer edges of the tubes (Fig. 8d). In other cases, especially with individual tubes, numbers of nanocrystals tended to disperse along the sidewalls, presumably attaching to defect sites on the oxidized nanotube surface (Fig. 8c). The TiO2 system showed substantially greater coverage of the tubes with the nanoparticles (Fig. 8b). Carboxyl groups adsorb strongly onto bare titanium dioxide surfaces.1-75-1 Hence, larger numbers of these particles can interact with nanotubes and in effect, amide bond formation may not be required for an underivatized, uncapped TiO2-SWNT adduct to be directly produced. Indeed, we postulate that this is the reason for the enhanced coverage with TiO2 particles. Energy-dispersive X-ray analysis on both nanocomposite systems was consistent with the presence of appropriately functionalized nanocrystals attached to nanotubes as proposed. These patterns of nanocrystal clustering on the nanotubes were not observed in control experiments, without the use of carbodiimide linking agents, where the tubes themselves were found to remain totally separate from the nanocrystals.
SOLUBILIZATION OF NANOTUBES
Whereas fluorination tends to derivatize tubes and disrupt band structure of SWNTs, most experiments involving solubilization of nanotubes aim for the retention of important photophysical properties. Moreover, solubiliza-tion facilitates the placement of a huge array of functional groups onto SWNT surfaces for composite formation.
By visual inspection and quantification using UV-visible absorption spectroscopy, small-diameter SWNTs (ca. 0.7 nm), grown by a gas-phase catalytic process,[76-were found to be somewhat soluble in a number of organic solvents such as 1,2-dichlorobenzene, chloroform, 1-methylnapthalene, dimethylformamide (DMF), and tet-rahydrofuran (THF).[55] Laser-oven produced SWNTs (with diameters of about 1.2 nm) can be dissolved to some extent in DMF, N-methylpyrrolidinone, and hex-amethylphorphoramide.[77] Many strategies aimed at improving the solubility behavior of nanotubes involve the oxidation and purification of nanotubes; reports[78,79] have placed the total percentage of acidic sites, generated by such processing, in full-length SWNTs at a range of 13% and defect sites in the 5% range. Examples of defects in nanotubes include pentagon/heptagon Stone-Wales-type defects, associated with a rotation of a bond in the SWNT atom network, vacancies in the nanotube lattice, the presence of adatoms or carbon dimers on the side-walls, the occurrence of sp3-hybridized carbons, and pentagon-terminated caps.[80-82]
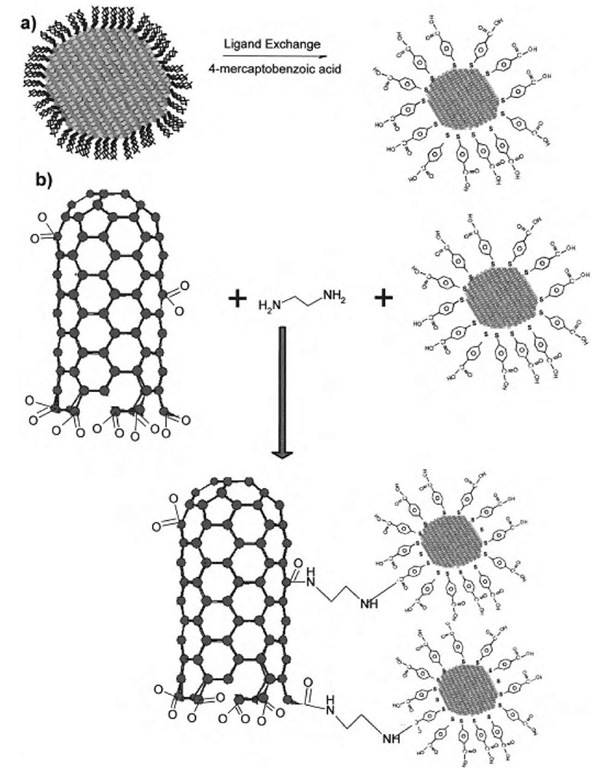
Fig. 7 Schematic of the addition of derivatized CdSe nanocrystals to oxidized SWNTs. Trioctylphosphine oxide (TOPO)-capped nanocrystals were prepared by established methods using organometallic precursors. (a) TOPO capping was substituted for a thiol ligand to form a carboxylic acid terminated CdSe nanocrystal. Substituted thiocarboxylic acids used included p-mercaptobenzoic acid, thioglycolic acid, and 3-mercaptopropionic acid. (b) The nanocrystals prepared in panel (a) were linked to oxidized SWNTs by an ethylenediamine linker in the presence of N-ethyl-N’-(3-dimethylaminopropyl)carbodiimide (EDC).
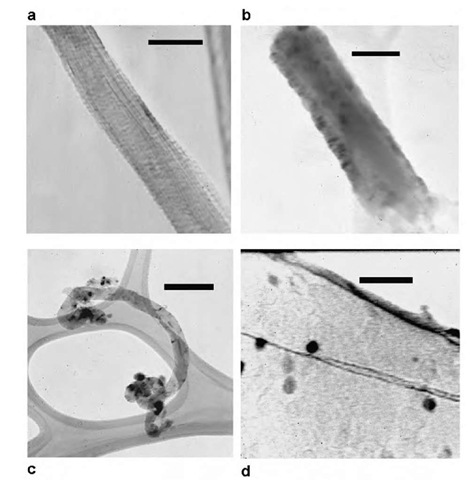
Fig. 8 TEMs of (a) a purified single-walled carbon nanotube bundle. Scale bar denotes 30 nm. (b) Oxidized SWNT bundle circumscribed by capped TiO2 particles. Scale bar is 45 nm. (c) Oxidized tubes linked to CdSe nanocrystals capped with 4-mercaptobenzoic acid. The crystallites are concentrated at the functionalized open ends, with some coverage on the sidewalls, likely on defect sites. Scale bar is 150 nm. (d) Oxidized nanotube linked to CdSe nanoparticles capped with 3-mercaptopropionic acid. Image at high magnification shows prolate nanocrystals scattered along the length of the tube. Scale bar represents 20 nm.
Indeed, metallic and semiconducting SWNTs were initially derivatized in organic solutions, such as chloroform, dichloromethane, aromatic solvents (including benzene, toluene, and chlorobenzene), and CS2, by converting acid-terminated, purified nanotubes with thio-nylchloride to generate acylchloride groups that could then be reacted with amines such as octadecylamine.[83] This work was later extended to amide formation from alkyl-aryl amines such as 4-dodecyl-aniline.[84] NMR spectra of the soluble SWNTs exhibited peaks because of the aliphatic and aromatic moieties, respectively. Raman spectroscopy indicated that nanotube structure was likely preserved. Solution-phase mid-IR analysis[85] shows that, for the reaction with octadecylamine, the weight percentage of the octadecylamino functionality is about 50%, at defect sites as well as the ends of SWNTs. Full-length dissolution[86] of purified SWNTs (containing carboxylic acid groups) was also achieved through an acid-base reaction by forming an octadecylammonium, SWNT-carboxylate zwitterion. AFM showed that the majority of the SWNT ropes were exfoliated into small ropes (2-5 nm in diameter) and individual nanotubes with lengths of several microns during the dissolution process.
Single-wall nanotubes, functionalized with esters[87] at defect sites [i.e., SWNT-COO(CH2)17CH3)], have been prepared, as determined by Raman and IR bands, leading to improved solubilization in solvents such as THF and CHCl3. Strong attachment of deuterium[88] to SWNTs in solution has been observed when deuterated ethanol is used as a coreactant in esterification reactions; results were obtained from 2H NMR and FT-IR measurements. Derivatization based on 1,3-dipolar cycloaddition of azomethine ylides, generated by the condensation of an a-amino acid and an aldehyde, has been used to solubilize tubes in most organic solvents.[89] Evidence of functionalization was further supported by observation of fluorescence in the adducts, as was also noted in SWNT-aniline systems.[90] SWNTs have also been functionalized with derivatized pyrene, via a similar reaction, namely the esterification of nanotube-bound carboxylic acids, to create adducts soluble in chloroform, THF, and toluene.[91] Fluorescence and fluorescence excitation results suggest that the tethered pyrenes form ”intramolecular” (intrana-notube) excimers. The pyrene monomer and excimer excitations are quenched by the SWNTs in a mechanism whereby the nanotubes serve as acceptors for excited-state energy transfers from the tethered pyrene moieties.
It has been proposed, for instance, that large organic groups assist in solubility by exfoliation of the bundles into individual tubes through the formation of intervening moieties that can overcome the intrinsic van der Waals forces[92] between these tubes. It is not surprising, given this improved solubility, that recent work has been performed on separating soluble SWNTs and zwitterion-functionalized shortened SWNTs by length, diameter, and chirality from THF using high-performance liquid chro-matography (HPLC) and gas chromatography techni-ques.[93,94] In addition, this body of solubilization work in stable, organic solvents has allowed Raman and near-IR spectroscopies to be used for the detection and identification of different species (i.e., diameters and metallicity) of SWNTs based on their Raman frequencies and vis-near IR electronic transitions.[95]
In water, a stable dispersion of full-length, well-separated individual tubes has been formed upon stabilization in gum arabic,[96] a water-soluble polysaccharide; adsorption is presumed to disrupt the intertube interactions in the crystalline ropes. Aqueous solubilization (in the range of 0.1 to 0.3 mg/mL) was also achieved by functionalizing SWNTs with glucosamine. In this case, grafting of the glucosamine to the tubes was achieved by reacting the amine with acyl chloride groups created on the SWNT surface.[97] Treatment of nanotubes with a 9:1 mixture of sulfuric acid and hydrogen peroxide for 30 min followed by sonication for varying lengths of time allows for the creation of carboxylate groups on defect sites and tube ends, which render the SWNTs water-soluble in different pH buffers. Electronic structure is maintained, as evidenced by absorption spectra, where the van Hove singularities are maintained.[28] There has also been a claim that SWNTs, sonicated and dispersed in 1% sodium dodecylsulfate buffer and glycerol, can be distributed to a certain extent by diameter and length simply by the application of an electric field, again implying that solubilization has consequences for rational manipulation of nanotubes.[98] Molecular dynamics simulations indicate that in water, polar functional groups attached to SWNTs are likely to be more energetically stable in extended configurations, whereas nonpolar functional groups will prefer to remain folded.[99]
To generalize and broaden the potential of functionalization, in our group, we have also solubilized oxidized SWNTs through the attachment of organic moieties in a large number of solvents of varying polarity.[100] In this case, we grafted a functionalized organic crown ether, namely 2-aminomethyl-18-crown-6, onto our SWNTs. The resultant, synthesized adduct yielded concentrations of dissolved nanotubes on the order of ~ 1 g/L in water as well as in methanol, according to optical measurements. These values are dramatic improvements over conventional dissolution behavior of unfunctionalized nanotubes. The nanotube-crown ether adduct can be readily redissolved in a number of different organic solvents, such as ethanol, 2-propanol, acetone, o-dichlo-robenzene (ODCB), dimethylformamide (DMF), tetrahy-drofuran (THF), dimethylsulfoxide (DMSO), ethyl acetate, and benzene, at substantially high concentrations. One implication of these results lies in further exploitation of the solution chemistry of these tubes for photophysical analyses in a number of previously untested solvents as well as for the generation of novel nanoscale architectures.
SWNT-POLYMER COMPOSITES
Single-wall nanotubes can also be solubilized in water by noncovalently associating them with linear polymers such as polyvinyl pyrrolidone and polystyrene sulfonate. In this case, the polymers rigidly and uniformly associate with the sides of nanotube, disrupting the hydrophobic interface with water and the intertube interactions within the tubular aggregates (Fig. 9). Nanotubes can then be ”unwrapped” by changing the solvent system.1-101-1 In another study, wrapping of polymer ropes (modified poly(p-phenylenevinylene) around the tube lattice occurs in a well-ordered periodic fashion, arising from a van der Waals interaction between the phenyl rings of the polymer and the hexagonal lattice of the nanotube; solutions of the polymer in toluene are thought to be able to suspend nanotubes ”indefinitely.”1-102-1 Based on Raman and absorption data, it is thought that the polymer interacts preferentially with nanotubes of certain diameters or a range of diameters. The attachment[103- of lipophilic and hydrophilic dendra, which are terminated with long alkyl chains and oligomeric poly(ethylene glycol) moieties, respectively, via amidation and esterification reactions, can render SWNTs and MWNTs soluble in common organic solvents, such as hexane and chloroform as well as water, to form colored homogeneous solutions. These functionalized tubes can be defunctionalized in homogeneous solutions under base- and acid-catalyzed hydrolysis reaction conditions.1-104-1 These functional polymer-nano-tube composites may have unique, favorable properties; for instance, SWNT-poly(metaphenylenevinylene) composites have nearly eightfold increase in electrical conductivity compared with just the polymer and have favorable photo- and electroluminescence properties as well.
Fig. 9 Molecular model of a (6,6) SWNT complex with poly(aryleneethynylene)s (PPE), showing interaction of the polymer backbone with the SWNT sidewall through p stacking.
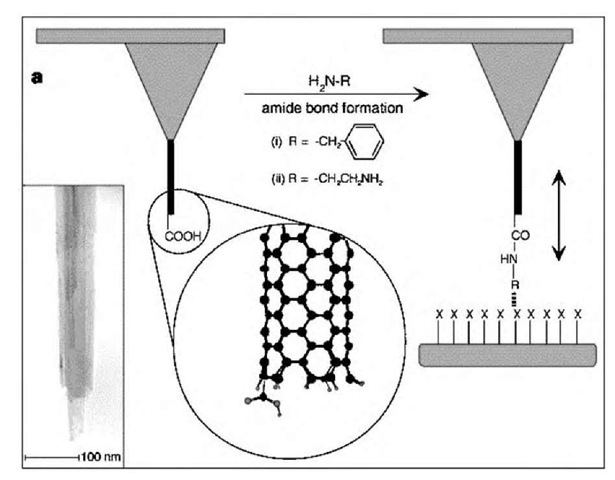
Fig. 10 Preparation of functionalized carbon nanotube tips. Diagram illustrating the modification of a nanotube tip by coupling an amine to a pendant carboxyl group, and the application of this probe to sense specific interactions with functional groups (X) of a substrate. The circular inset is a molecular model of a single nanotube wall with one carboxyl group at the tip end. Inset: TEM image showing the open end of a shortened nanotube tip.
ADDITIONAL CHEMICAL FUNCTIONALIZATION STRATEGIES
Single-wall nanotubes can also be modified by a number of other methodologies. Sonication of tubes in a mono-chlorobenzene solution of poly(methyl methacrylate) (PMMA) created SWNTs with many defects in the side-walls when burned in oxygen.[106] Using radio frequency glow-discharge plasma activation,[107] amino-dextran chains have been immobilized onto acetaldehyde-treated aligned SWNTs through a Schiff-base formation and reductive stabilization with sodium cyanoborohydride, while periodate-oxidized dextran-fluorescein isothiocya-nate (FITC) chains have been chemically grafted onto ethylenediamine-derivatized SWNTs via the same reaction. SWNTs can also be derivatized by bombardment1-108,109-1 with CH3 and CF3 radicals impacting with incident energies of 10, 45, and 80 eV, as was confirmed by X-ray photoelectron spectroscopy and scanning electron microscopy.
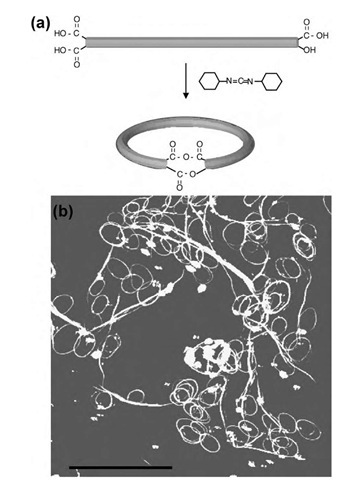
Fig. 11 (a) A possible scheme for nanotube ring closure with N,N’-dicyclohexylcarbodiimide (DCC). (b) AFM images of closed ring systems. The scale bar is 2 mm.
Chemical functionalization of SWNTs attached to conventional atomic force microscopy (AFM) probes was also demonstrated as a methodology yielding high-resolution, chemically sensitive images on samples containing multiple chemical domains.1-10,74,110-1 Moreover, when these probes were derivatized with amine, carboxy, and biotin groups at their ends with domains of similarly defined functional groups localized on the surface, they could be used to measure specific and definable interactions in a variety of solvent media (Fig. 10).
MANIPULATION OF TUBES
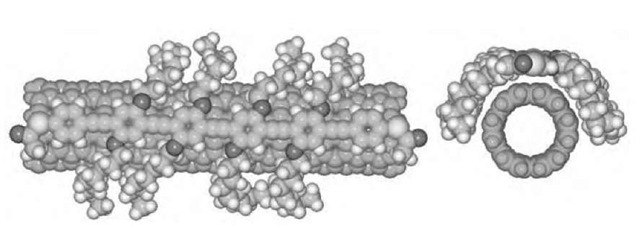
Although it appears that oxidized SWNTs have a tendency to stack and form larger aggregates,1-111-1 interesting nano-scale architectures can be created by judicious manipulation of the chemistry of these tubes. For instance, lightly etched SWNTs have been chemically reacted1-112-1 to form rings, with an average diameter of 540 nm (Fig. 11). The same research group created a hollow spherical ring made of nested SWNTs.[113- To accomplish this geometry, SWNTs were first adsorbed onto amine-terminated silica gels in solution, a step that determined the nature of the nested network. Subsequent drying and readsorption cycles were used to grow the nest layer-by-layer through nanotube-nanotube interactions. Ultimately, the silica gels were etched away to give hollow cages.
Carboxy-terminated SWNTs have been assembled onto amino-terminated gold surfaces via electrostatic interactions, with the aid of dicyclohexylcarbodiimide condensation agents.[114- Similar chemical strategies were employed to adsorb SWNTs onto silver surfaces.1-115-1 Work has also been completed on depositing tubes on chemically functionalized nanolithographic patterns. Creation of these assemblies is critical because these strategies allow for precise positioning of nanotubes in specific locations and orientations; consequently, there is now the potential of creating simple nanostructures with SWNTs and of connecting these to other structures. This is crucial to the fabrication and development of simple electrical circuits with tubes and hence, provides for a means of measuring electron transport characteristics in these systems.1-116-1 Recently, it has also been found1-117-1 that ozonized SWNTs can be assembled onto amine-terminated self-assembled monolayers (SAMs) as well as physically adsorbed via layer-by-layer deposition with bridging of metal cations, i.e., Fe+3 on carboxylate-terminated SAMs or Cu+2 on thiol-terminated SAMs. A further advance has come from a report of room-temperature assembly of directional SWNT strings across polymer microchannels.[118-
CONCLUSION
Rational nanotube functionalization provides for the potential of the manipulation of nanoscale properties in a predictive manner. In many cases, it may also render tubes soluble and stable in aqueous and organic solution, thereby enabling further exploitation of their wet chemistry and investigation of their photophysical properties. In addition, derivatization allows for a number of site-selective nanochemistry applications such as the possibility of self-assembly of nanotubes with tailorable electronic properties, important for advances in molecular electronics. Other derivatized nanotube adducts also show the potential for novel charge transfer characteristics, the development and understanding of which have implications for photocatalysis as well as for scanning probe microscopy with functionalized tips. Finally, chemical manipulation of SWNTs is critical for hierarchical buildup of these nanomaterials into novel architectures, such as nanocomposites and nanocircuits, with unique structural properties.