INTRODUCTION
Fullerenes are the most exciting new set of chemical structures discovered in the last part of the 20th century. The advent of these novel structures has led to fascinating new scientific questions and to intriguing potential applications. This article provides an overview of the structures of the common members of the fullerene class of carbon allotropes and introduces the essentials of fullerene reactivity. Specific types of reactivity discussed herein include reactions of fullerenes with nucleophiles and electrophiles, oxidations and reductions, as well as reactions with metals and cycloaddition reactions. A glimpse of the potential applications of these fascinating molecules is also provided. The focus will be on the chemistry of C60 as it is the most available and the best-understood member of an expanding set of chemical structures. The chemistry of C70 is discussed briefly as well, demonstrating how strain directs the reactivity of this less-symmetrical structure.
STRUCTURE
Fullerenes are hollow clusters of carbon atoms. The name fullerene is a shortened form of buckminsterfullerene, a name that pays homage to the designer of the geodesic dome.a The structure of C60 is reminiscent of that famous dome, being a near-perfect sphere formed from a set of fused rings in the same manner as a soccer ball. C70 is similar, having the same general structure with an extra ”belt” of 10 carbon atoms. Common members of this class of structures include C60, C70, C76, and C84, although small amounts of larger structures (”higher fullerenes”) are formed along with the more common species. As the number of carbon atoms increases, the number of possible structures increases, and indeed multiple isomers of several of the higher fullerenes have been observed.b New fullerene species have been prepared from other fullerenes, albeit in low yield.c
The C60 cage (Ih symmetry) measures 7 A across. The C70 ”rugbyball” shape (D5h symmetry) is 7 A across and 10 A long. These highly curved structures place carbon in a strained geometry. Trivalent carbon (sp2 hybridization) typically leads to a planar geometry. In fullerenes, the bonding arrangement at carbon is far from planar—the three bonds form a shallow pyramid.[4'5] The strain in C60 is significant (over 400 kcal/mol) and is responsible for much of the reactivity of fullerenes.
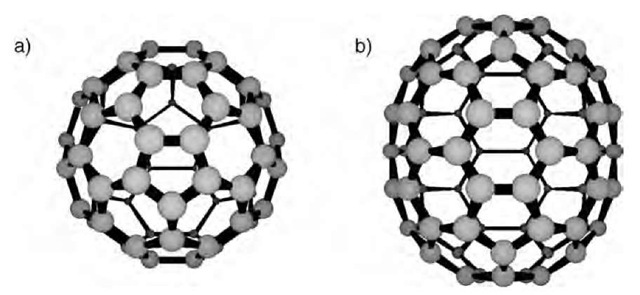
The bonding pattern in these molecules may be better illustrated through a ”Schlegel” diagram, which is a flattened projection of the three-dimensional structures shown in Fig. 1. In these diagrams, the pattern of 5-membered and 6-membered rings is easily seen.
Only the pattern of a bonds is shown in Figs. 1 and 2. There is an extensive (and critically important) set of p bonds that involves every carbon atom in the molecule. It is clear from the reactivity of C60 as well as from theory that these bonds are not localized between pairs of carbon atoms, but are instead delocalized over the surface of the fullerene cage. There is some debate over whether fullerenes can be properly classified as aromatic.[7] Aromaticity is normally associated with significant electronic ring currents, diminished reactivity, and diminished bond length alternation. Fullerenes display a degree of bond length alternation. The bonds that form the fusion of a 6-membered ring with a 5-membered ring are somewhat longer (bond length of 1.46 A) than the bonds that form the fusion of two 6-membered rings (1.40 A), but this amount of bond length alternation is also found in some molecules commonly considered to be aromatic (Fig. 3).
Fullerenes are much more reactive toward addition reactions than are classical aromatic compounds such as benzene. This likely reflects the relatively poor overlap of p-orbitals (splayed apart as a result of the curvature of structure), as well as the tremendous release of strain that results from conversion of carbon atoms from highly strained sp2 geometry to a much less strained sp3 geometry. Ring currents in fullerenes are segregated into opposing diamagnetic and paramagnetic currents that sum to 0 in the case of C60 and to small values for the other fullerenes. It is clear that traditional notions of aromaticity and indeed some of the traditional hallmarks of aroma-ticity, which were originally developed for planar, monocyclic systems, are not easily applied in the case of these spherical, polycyclic structures. A novel 2(N +1)2 rule has been proposed as a guideline for aromaticity in spherical systems.[8]
Fig. 1 The two most common fullerenes: a) C60 and b) C70.
C60 has three degenerate lowest unoccupied molecular orbitals (LUMOs) and can be reduced to the C6cT anions, where n is 1-6. The reduction can be achieved electro-chemically, by electron transfer from various anions, or by direct reduction with metals. Reviews on electron-transfer reactions of fullerenes[14] and of the preparation and properties of the anions (fullerides) and the less well-known cations (fullerenium ions) have been published.[15]
Synthesis
These beautiful molecules were first detected in the plume above a laser-evaporated carbon target and have also been formed on a large scale in electric arcs between carbon electrodes and in particular types of flames.[9-11] The precise mechanism by which fullerenes form is not known, but they seem to occur during the condensation of carbon plasmas. There has been a total synthesis of C60 reported,[12] but the ease, low cost, and scalability of arc and of flame methods clearly make these the preferred routes. In essentially all cases, the dominant fullerene formed is C60, and the amounts of the higher fullerenes (C70 and higher) diminish rapidly, to the point where miniscule amounts of fullerenes larger than C84 are formed. In 2003, a large-scale facility dedicated to fullerene production was established, using the flame method.
Fig. 2 Planar projection of C60, showing the pattern of 5- and 6-membered rings.
Purification of fullerenes is usually accomplished by chromatography, and numerous methods have proven effective. On large scales, short silica/carbon columns are most appropriate. On smaller scales, sublimation can be used to produce highly pure, solvent-free fullerenes.
Fullerenes are typically dark, sometimes lustrous, solids. The liquid phase has not been observed, but the solids sublime well under high vacuum. The solubility of fullerenes is negligible in hydrocarbon solvents, but reasonable in aromatic and halogenated aromatic sol-vents.d Larger fullerenes are typically less soluble than smaller ones.
CHEMICAL REACTIVITY
When macroscopic amounts of fullerenes became available for study around 1990, it did not take long to discover that these molecules were quite reactive.6 Because full-erenes themselves have no chemical groups available for substitution, they can react only in addition reactions.[17] The driving force for these reactions is provided (in part) by the release of strain from pyramidalization. The chemistry of fullerenes has turned out to be very rich.f
Fig. 3 A common type of drawing of C60, showing double bonds at the site of the shortest bonds and not showing the back side of the ball.
Hydrogenation
Hydrogenation of fullerenes is easily accomplished under a variety of conditions. The simplest hydrogenated fullerenes, C60H2 and C70H2 (Fig. 4), have been made by reduction with BH3, with Zn, with diimide, and with other reagents.g In both of these cases, the two hydrogen atoms are bonded to adjacent carbons, as this arrangement is more stable than arrangements that place the hydrogen atoms farther apart. Interestingly, C60H2 is a remarkably acidic compound for a hydrocarbon, with a pKa below 5.
As expected, more highly hydrogenated compounds can be obtained, and compounds out to C60H36 have been prepared, using transfer hydrogenation, Li/NH3, or high-pressure catalytic hydrogenation. While C60H18 is isolated as a single species, C60H36 is isolated as a mixture of isomers. The structure of C60H18 has been established as having a ”turtle-shell” shape (Fig. 5).
Oxidation
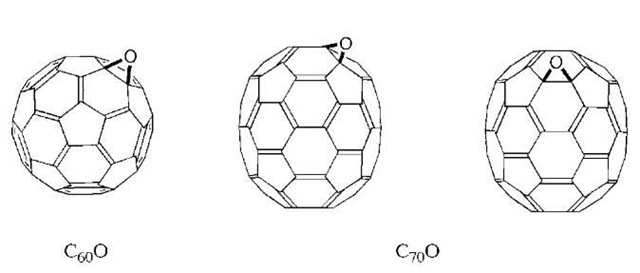
Treatment of C60 with most oxidizing agents results in the formation of C60O. This compound is less soluble than C60 itself and less chromatographically mobile. Multiple oxidations occur with excess oxidizing agent, and multiple isomers of the C60Ox product are formed. These oxides are increasingly insoluble and difficult to characterize. Oxidation of C70 is somewhat more complex, as multiple isomers of the monoadduct are possible. The two predominate isomers formed are shown in Fig. 6.
Fig. 4 a) C6oH2 and b) C70H2.
Cycloadditions
Fullerenes react readily in a variety of different cycload-ditions.h These reactions often proceed at room temperature, although multiple additions occur. In most cases, a single cycloaddition to C60 will produce a single isomer of monoadduct, but a second cycloaddition produces a mixture of isomeric diadducts. Reactions on less-symmetrical higher fullerenes are even more problematic. In practice, reactions are usually limited to less than 50% conversion to maximize the yield of monoadduct.
A number of different [2+2] cycloadditions have been reported. For example, benzyne adds in a [2+2] manner to produce a new 4-membered ring fused to C60 (Fig. 7). This reaction is thermal, although there are analogous photochemical reactions between fullerenes themselves, for example, the photopolymerization of C60. While strong dienophiles will react with C60, they do so in a [2+2] manner rather than the [4+2] manner of the classical Diels-Alder reaction.
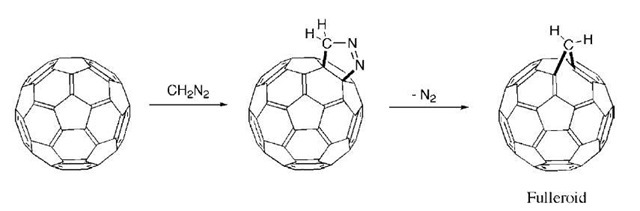
A number of different [3 + 2] cycloadditions have been reported, leading to a rich variety of products. Nitrile oxides add to C60 to form isoxazolines, azomethine ylids add to form pyrrolidines, and diazoalkanes add to form pyrazolines. The latter case is one of the most important reactions in fullerene chemistry, and the C60-pyrazoline adduct is thermally unstable and decomposes to new products. In the case of Ph2CN2, the primary product is the methanofullerene. However, in the case of CH2N2, the product is a novel compound C61H2, known as a homofullerene or a ”fulleroid.”1 Fulleroids are fullerenes that have been ”inflated” by the addition of a carbon atom and the opening of one of the original C—C bonds in the cage. The opened bond is typically the bond that formed the fusion of a 5-membered ring with a 6-membered ring (Fig. 8).
Fig. 5 The structure of C60H18.
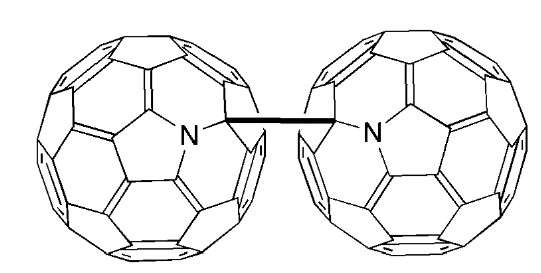
Similar chemistry occurs with alkyl azides, leading to the formation of azafulleroids. Azafulleroids have served as the starting material for the formation of C59N-based species, a rare example of a fullerene cage with a hete-roatom replacing one of the carbon atoms[26] (Fig. 9).
Fullerenes undergo a number [4+2] cycloadditions, with the fullerene functioning as the 2-electron component. Reaction with dienes such as cyclopentadiene yields adducts, and, as is often the case, multiple additions can occur.
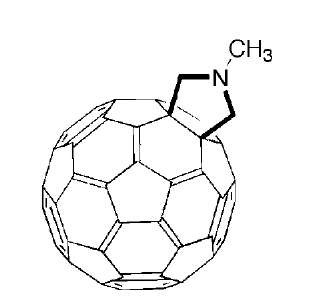
Of the various 1,3-dipolar cycloaddition reactions that have been demonstrated on fullerenes, the addition of azomethyne ylids has proven to be particularly versatile. This reaction converts a fullerene into a fulleropyrrolidine (Fig. 10). The nitrogen atom provides a convenient point of attachment for a host of different groups.
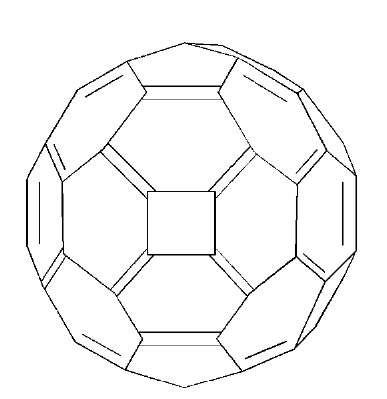
A tandem [4+4]/[2+2+2] cycloaddition route has been used to generate a novel fullerene (C62) that is not formed in significant amounts in arc reactors (Fig. 11).
Fig. 6 The monooxides of C60 and C70.
Fig. 7 The [2 + 2] cycloaddition of benzyne to C60.
Addition of Nucleophiles
Fullerenes react in a manner not unlike electron-deficient alkenes. Strong nucleophiles add readily and often add repeatedly if conditions are not carefully controlled. Examples of carbon nucleophiles that react with C60 include alkyl lithium reagents, alkyl magnesium (Grignard) reagents, acetylide anions, and cyanide. Addition of several equivalents of nucleophile is a common side reaction. Organocuprates also add readily, resulting in a symmetrical cyclopentadienyl pattern. Heteroatom nucleophiles, including amines, are also known to add to C60.
One of the most useful reactions in all of fullerene chemistry is the addition of dialkyl bromomalonate anion. This reaction, often called the Bingel reaction, results in formation of a cyclopropane (Fig. 12). Most likely, this reaction involves addition of the enolate nucleophile, followed by displacement of the bromide ion by the resulting fulleride anion. This reaction has produced Td-symmetrical species that display luminescence. Luminescence from fullerenes and fullerene derivatives is uncommon.[27]
Addition of Radicals
Radicals add readily and repeatedly to fullerenes.Alkyl radicals can add over 30 times to C60. Sulfur- and nitrogen-based radicals have been observed to add to fullerenes. The tendency for multiple additions and multiple isomers has limited the synthetic utility of this reaction. However, radical addition to fullerenes has been used as method for covalent incorporation of fullerenes into polymers.
Addition of Electrophiles
Halogenation of fullerenes is easily accomplished with a variety of reagents. The resulting compounds are reactive under a variety of conditions, establishing the halogenated compounds as useful synthetic intermediates. Addition of F2, Br2, and Cl2 to C60 are all known, but addition of I2 is not favorable. Reaction of C60 with neat Br2 produces C60Br8 and C60Br24 (Fig. 13).[29] The addition is reversible, as both products loose Br2 upon heating.
Fig. 8 Formation of a fulleroid via addition of diazomethane to C60.
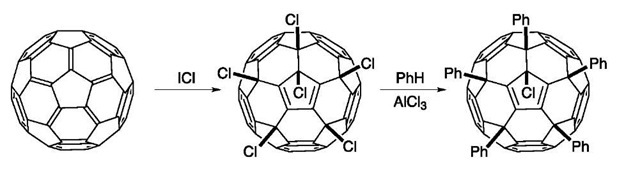
Chlorination with ICl produces C60Cl6, an interesting compound with an isolated cyclopentadienyl substructure. The presence of chlorines opens up new chemical possibilities, and these atoms have been substituted by methoxide and by aromatic rings.[30] Dissolving C60Cl6 in benzene with AlCl3 brings about a classical Friedel-Crafts reaction, producing C60Ph5Cl (Fig. 14).[31] This compound has been used to make metal-cyclopentandi-enyl complexes.
Fluorination has resulted in a number of different highly fluorinated species (up to C60F48),[32] and these are typically reactive toward nucleophiles, including weak nucleophiles such as water. In many cases, the addition patterns seen in fluorination reactions mimic the pattern seen in hydrogenation.
Fuming sulfuric acid adds to C60 to produce cyclic sulfates. These can be hydrolyzed to produce polyhy-droxylated compounds known as fullerols. These compounds are usually isolated as complex mixtures, containing species with different numbers of hydroxyl groups and numerous isomers for each molecular formula. Fullerols are notable as one of the few classes of fullerene derivatives that are commonly water-soluble.
Electrophilic Substitution
The chemistry of most aromatic hydrocarbons is characterized by substitution reactions, in which an attacking electrophile replaces a peripheral hydrogen. The lack of peripheral groups (the lack of a periphery!) makes it impossible to do a true substitution reaction on a fullerene, but fullerene derivatives can participate in these reactions. The halides have proven to be useful in electrophilic aromatic substitution reactions. Dissolving C60Cl6 in benzene with AlCl3 brings about a classical Friedel-Crafts reaction, producing C60Ph5Cl.
Fig. 9 The C59N dimer.
Multiple Additions
While a single isomer results from addition of one group to C60, addition of a second group can result in large number of different isomers. Two isomers are the most common, but in most cases, complex mixtures of many isomers result.
A template-directed approach has been used to address this problem. This approach relies on the initial addition that connects a template to the fullerene. The template bears reactive groups that lead to additional reactions on the fullerene, but only at sites that can be reached by the template.-1
Metal Complexes
Fullerenes form complexes with a wide array of transition metals,[34,35] essentially always in a 2 manner.k These complexes are often very insoluble, but a number have been crystallographically characterized. Complexes with platinum and iridium are common, and a typical example is shown in Fig. 15.
Alkali metals and alkaline earth metals generally undergo electron-transfer reactions with fullerenes, leading to MxC60 species, where x depends on how strongly reducing the metal is. In solution, the exact species formed depends on the metal and on the solvent.
The most novel aspect of the organometallic chemistry of fullerenes is the chemistry of endohedral fullerenes.
Fig. 10 A fulleropyrrolidine.
Endohedral complexes of fullerenes are composed of one or more atoms inside of a carbon shell. Such species are commonly denoted as ”M@C60,” meaning atom M inside C60.[37,38] There is very little free space inside of a fullerene, so only individual atoms or small groups of atoms can be accommodated.
Endohedral fullerenes can be prepared in a number of different ways. It is possible to drive small inert gas atoms directly into the interior of a fullerene. This is the common synthetic route for species such as 3He@C60 and 3He@C70. This procedure requires high temperature (>600°C) and pressures (>2000 atm) and results in low (< 0.1%) incorporation.1-39-1 Regardless of the difficulty, these 3He complexes have proven invaluable for the 3He NMR signal, which has been used to determine the number of isomers of a fullerene (or fullerene derivative) present in a sample and as a probe of the internal electronic environment.
A second route to endohedral fullerenes is to form the fullerene around the endohedral atom(s). This is most commonly performed by using a metal-doped carbon rod in an arc reactor. The resulting endohedral complexes are then isolated from the resulting soot and purified by chromatography. A wide range of endohedral complexes has been formed by this method, and the carbon shell formed around the metal is often (usually) not a C60 or a C70 shell.
Fig. 11 The structure of C62.
Fig. 12 Cyclopropanation of C60.
The most common members of this class of compound are species such as La@C82. Much larger shells and much smaller shells have been observed (La3@Ci06, U@C28). Multiple atoms can be encapsulated, including a mixture of metals and nonmetals (Sc3N@C82).
The Chemistry of Higher Fullerenes
Higher fullerenes (C70 and larger fullerenes) have received much less attention than has C60.[40,41] The limited availability and high price of these molecules have made it difficult to undertake extensive studies of their chemistry. Thankfully, the reactivity of the higher fullerenes is very similar to that of C60. However, because C70 and the higher fullerenes are less symmetrical than C60, there are more options for the site of the first addition, for the second addition, and so on. Hirsch-Bingel reactions on C70 have been used to identify the most reactive site, the second reactive site, and so on.[42] As expected, the most reactive site on C70 is the C=C bond between the most pyramidalized carbons in the molecule. The second most reactive site is typically at the opposite pole of the molecule. The last to react are usually the carbons closest to the equator.
Fig. 13 C60Br24, showing one side of the structure.
Fig. 14 Preparation and reactions of C60Cl6.
There are exceptions to this rule. Addition of chlorine, as well as reduction with Zn(Cu), results in addition near the equatorial belt. This pattern may result from a different mechanism (radical?) than is operating. As a result, reactions on the poles and near the equatorial belt are known.
APPLICATIONS
A variety of applications have been proposed for full-erenes based on the potential exploitation of the electronic or optical properties. The quantum yield for production of the triplet excited state of C60 is nearly unity, and C60 is an efficient sensitizer for producing singlet oxygen. The high molar absorptivity and absorption at the long-wavelength end of the visible spectrum, coupled with being an efficient sensitizer, makes fullerenes good candidates in photodynamic therapy. Fullerenes exhibit nonlinear optical behavior, and so optical limiting applications are possible.[43] Fullerene derivatives have also been used in enzyme inhibitors, and fullerene derivatives have shown activity against a number of tumor cells lines.[44] Fullerols have shown some promise as MRI contrast agents.
Fig. 15 A typical transition metal complex of C60, (Ph3P)2-PtC60.
Most of the potential applications lay in the electronic and materials areas. Transistors have been made from C60 films and from C70 films.[45] Much excitement was generated when the superconductive M3C60 species were discovered, and these fulleride films and Tc’s as high as 33 K have been measured in Cs2RbC60. While some advances beyond 33 K have been reported, to date, the Tc’s of fullerene-based superconductors have not surpassed copper oxide-based materials.
CONCLUSION
Fullerenes are closed shells formed of carbon atoms. The chemistry of these novel forms of carbon is similar to electron-deficient alkenes, in that the fullerenes react with nucleophiles faster than with electrophiles. The chemistry of fullerenes is largely addition chemistry, and a host of reagents is known to add to C60 and other fullerenes. Most addition reactions will proceed multiple times, and conversions are typically limited to ~ 50% to produce the highest yield of monoadduct. Multiple additions generally result in complex mixtures of isomers. Higher fullerenes (C70 and larger) pose even more daunting regiochemical problems. Template-directed methods can help produce diadducts, triadducts, and higher adducts with predictable regiochemistry.




![The [2 + 2] cycloaddition of benzyne to C60. The [2 + 2] cycloaddition of benzyne to C60.](http://what-when-how.com/wp-content/uploads/2011/03/tmp1CF15_thumb_thumb.jpg)