INTRODUCTION
Nanoparticles have no exact definition, but they are aggregates of atoms bridging the continuum between small molecular clusters of a few atoms and dimensions of 0.2-1 nm and chunks of solid containing millions of atoms and having the properties of macroscopic bulk material. In water, nanoparticles include colloids; in air, they include aerosols. Nanoparticles are ubiquitous. We pay to have them. We pay more to not have them. They occur as dust in the air, as suspended particles that make river water slightly murky, in soil, in volcanic ash, in our bodies, and in technological applications ranging from ultratough ceramics to microelectronics. They both pollute our environment and help keep it clean. Microbes feast on, manufacture, and excrete nanoparticles.
Understanding nanoparticle formation and properties requires sophisticated physics, chemistry, and materials science. Tailoring nanomaterials to specific applications requires both science and Edisonian inventiveness. Applying them to technology is state-of-the-art engineering. Tracing their transport and fate in the environment invokes geology, hydrology, and atmospheric science. Applying them to improving soil fertility and water retention links soil science and agriculture to surface chemistry. Understanding their biological interactions brings in fields ranging from microbiology to medicine. Probing the impact of nanoparticles on humans and of human behavior on the production and control of nanoparticles requires the behavioral and social sciences, e.g., in dealing with issues of automotive pollution. The purpose of this review is to describe some of the unique features of nanoparticles and to discuss their occurrence and importance in the natural environment.
Although we often think of the natural environment as that part of the planet which we can see, a somewhat broader definition includes the ”critical zone”: the atmosphere, hydrosphere, and shallow portion of the solid earth that exchange matter on a geologically short time scale, on the order of tens to thousands of years. This critical zone affects us directly, and our activities influence it. Because of the active chemical reactions continuously taking place in the critical zone, and because its temperatures and pressures are relatively low and it is dominated by water, solids are constantly being formed and decomposed.
Many of these solids start out as nanoparticles; many remain so. In a yet broader sense, our entire planet from crust to core, the solar system, and the galaxy are part of our environment.
PHYSICAL CHEMISTRY OF NANOPARTICLES
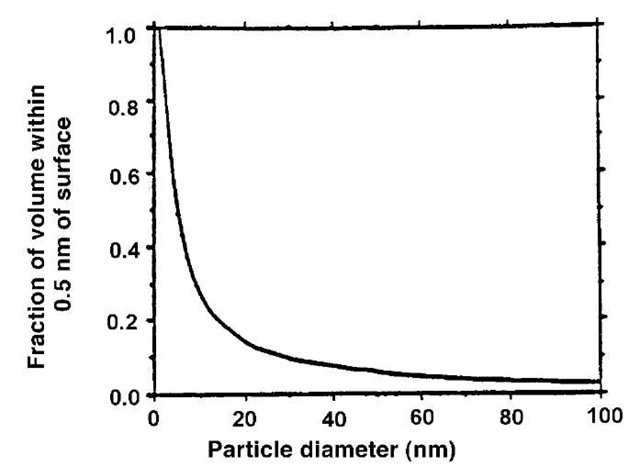
A major feature of nanoparticles is their high surface-to-volume ratio. Fig. 1 shows the volume fraction within 0.5 nm of the surface for a spherical particle of radius r. One can think of this fraction either as the fraction of atoms likely to be influenced by processes at the surface, or as the fraction of the volume of a material that could be taken up by a 0.5-nm coating of another material. In the first case, because the surface dominates chemical reactivity, the increased surface to volume ratio means that nano-particles dominate chemical reactions. In the second case, the ability to carry a substantial coating offers a mechanism for the transport of nutrients or pollutants.
Many oxides are polymorphic, exhibiting several crystal structures as a function of pressure and temperature. Often, nanosized oxide particles crystallize in structures different from that of large crystals of the same composition.[1] Examples are y-Al2O3, a defect spinel rather than a-Al2O3, corundum, g-Fe2O3, the defect spinel maghemite rather than a-Fe2O3, hematite, and the anatase and brookite forms of TiO2 rather than rutile. From arguments based on transformation sequences and the occurrence of phases, it was long argued that there may be a crossover in phase stability at the nanoscale if the structure which is meta-stable for large particles has a significantly lower surface energy.[2] This has been proven for alumina and titania in recent calorimetric studies (Fig. 2).[3,4] The resulting transformation enthalpies and surface energies, and those of other related systems are shown in Table 1. Another interesting feature is that the hydrous phases AlOOH boehmite and FeOOH goethite have significantly lower surface energies than their anhydrous counterparts, Al2O3 and Fe2O3.[5,6] Whether this is a general feature of hydrous minerals with hydroxylated surfaces is not yet known.
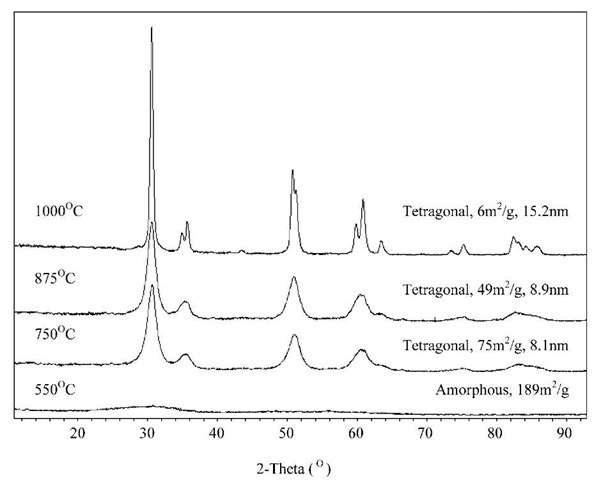
As particles become less than about 10 nm in size, their X-ray diffraction patterns are broadened sufficiently that they begin to appear ”X-ray amorphous” (Fig. 3). This term lacks exact definition. High-resolution electron microscopy may still detect periodicity, and short-range order is certainly present.[7] The identification of structure in 1-10 nm particles is very difficult, and phases are empirically described as, for example, ”two line ferrihy-drite,” based on x-ray diffraction patterns.
Table 1 Energetic parameters for oxide and oxyhydroxide polymorphs
Fig. 2 (a) Enthalpies of alumina polymorphs as a function of surface area. (From Ref. [3].) (b) Enthalpies of titania polymorphs as a function of surface area. The heavy lines show the stable polymorphs in each size range.
Table 1 Energetic parameters for oxide and oxyhydroxide polymorphs
|
Formula |
Polymorph |
Metastability (kJ/mol) |
Surface energy (J/m2) |
|
Al2O3a |
Corundum (a) |
0 |
2.6 |
|
Spinel (g) |
13.4 |
1.7 |
|
|
Fe2O3b |
Hematite |
0 |
0.8 |
|
Maghemite |
20 |
0.8 |
|
|
TiO2c |
Rutile |
0 |
2.2 |
|
Brookite |
0.7 |
1.0 |
|
|
Anatase |
2.6 |
0.4 |
|
|
AlOOHd |
Diaspore |
0 |
? |
|
Boehmite |
4.9 |
0.5 |
|
|
FeOOHb |
Goethite |
0 |
0.3 |
|
Lepidocrocite |
0.3 |
NANOPARTICLES IN SOIL AND WATER
Soil is a complex aggregate of inorganic, organic, and biological material.[9] Its constituents of largest size are rocks and gravel, small animals, plant roots, and other debris. Smaller mineral grains, clumps of organic matter, and microorganisms make up an intermediate size fraction. The smallest particles, ranging into the nano-scale, are clays, iron oxides, and other minerals. These are often heterogeneous and coated by other minerals and organic matter. The entire composite is porous and hydrated. The percolation of water in soil transports both nanoparticles and dissolved organic and inorganic species. The texture and porosity, as well as the chemical composition and pH, are crucial to biological productivity. The surfaces of nanoparticles provide much of the chemical reactivity for both biological and abiotic processes.
Major aluminosilicate minerals in soils include clays, zeolites, and poorly crystalline phases (Table 2). These can change their water content in response to ambient conditions, often swelling in wet seasons, and shrinking in dry seasons. These nanophase materials are major controllers of soil moisture and permeability. Iron and manganese oxides are another class of major sol minerals.
Fig. 3 Powder X-ray diffraction patterns of sol-gel zirconia heated at various temperatures. The structure and average particle diameters are indicated.
Their extensive polymorphism at the nanoscale makes them highly variable. They sequester and/or transport and make available the essential plant nutrient iron, as well as other essential transition metals (cobalt, copper, zinc, etc.). They frequently carry coatings of other metal oxides and oxyhydroxides, including toxic metals such as lead and chromium. They also frequently have organic coatings. Sulfates, including the jarosite-alunite family of hydrated [(K, Na), (Al, Fe)] sulfates, are another important constituent. In alkaline and arid environments, other sulfates and halides form, and their formation, dissolution, and transport is a major issue in heavily irrigated regions. How much these processes are controlled by nanoscale phenomena is not known.
Groundwater is constantly in touch with soil and rock, and minerals are dissolving and precipitating as it flows. The load of fine sediments in streams and groundwater can be substantial, especially during spring floods. The Missouri River is called ”the Big Muddy” because of its load of particulate matter, a large fraction of which is of nanoscale dimensions. The yearly flooding of the Nile, depositing fertile soil with its large nanoparticle content, made ancient Egyptian civilization flourish. Today, one of the major concerns of our system of dams, especially in the arid western United States, is interference with the normal cycle of sediment transport and ”silting up” of the lakes behind the dams. Silt is partly nanoparticles.
Table 2 Major soil minerals and constituents
|
Type |
Composition |
Structures |
|
Clay |
Hydrated aluminosilicate |
Layered |
|
Zeolite |
Hydrated aluminosilicate |
Three-dimensional porous |
|
Salts |
NaCl, Na2SO4, CaSO4 |
Ionic crystals |
|
Carbonates |
CaCO3-MgCO3-FeCO3 |
Calcite, dolomite, others |
|
Allophane |
Hydrous aluminosilicate gel |
Amorphous |
|
Iron oxides |
Fe2O3, FeOOH |
Various polymorphs |
|
Aluminum oxides |
AlOOH, Al(OH)3 |
Various polymorphs |
|
Quartz |
SiO2 |
Quartz |
|
Manganese oxides |
Mn2O3, MnOOH, MnO2 |
Various polymorphs |
|
H2O |
H2O |
Water, ice, vapor |
|
Organics |
C-H-N-O |
Large surface area amorphous colloids |
|
Jarosite-alunite |
Alkali (Fe, Al) sulfates |
Ionic double salts |
Contaminants and pollutants in water can be transported as aqueous ions (dimensions <0.5 nm), as molecular clusters (0.5-2 nm), as nanoparticles (2-100 nm), as larger colloids (100-1000 nm), and as macroscopic particles (>1 ^m). These size range distinctions are rather arbitrary and serve to illustrate the continuity between the dissolved and the solid state. Several examples illustrate this complexity. Aluminum oxyhydroxide particles can transport transition metals such as nickel, cobalt, and zinc, seemingly as adsorbed coatings. Initially thought to be loosely bound metal complexes at the surface of the aluminum oxyhydroxide mineral grain, these are now realized to be precipitates, only a few atomic layers thick, of mixed double hydroxides of the hydrotalcite family, in which anions such as carbonate play an essential role.[10] The transport of plutonium through groundwater is a concern in old plutonium processing facilities such as the Hanford, WA atomic energy reservation, in the Nevada nuclear test site, and in the planned nuclear waste repository at Yucca Mountain, Nevada. There remain questions of permeability and the adhesion of particles to the rock and engineered barrier walls, of colloid transport, of biological transport, and of mineral precipitation which can change the rate of progress of a contamination plume. Linking laboratory scale, field scale, and simulation studies of nanoparticle transport is an essential area of research for understanding radioactive and chemical contamination and geologic processes involving uranium and other actinides.[11]
When particles are below 5 nm in size, several other effects must be considered. Whereas for larger particles, most of the atoms are in specific planes or faces, for smaller ones, an increasing number of surface atoms must sit at the intersection of facets, in presumably even higher energy sites. An alternate, more macroscopic way of describing this is to consider the surface as curved, rather than as a series of planes. Then the surface energy per unit area is no longer a constant, but potentially increases quite rapidly with decreasing particle size. This unfavorable energy may be relaxed by the adsorption of various molecules on the surface, and there is evidence that the adsorption coefficient of organics rises steeply at very small particle size.[12]
The flocculation of colloids depends on the surface charge; the pH of which the surface is neutral is the ”point of zero charge.”[13] Does this depend on particle size? This is an area of active research.
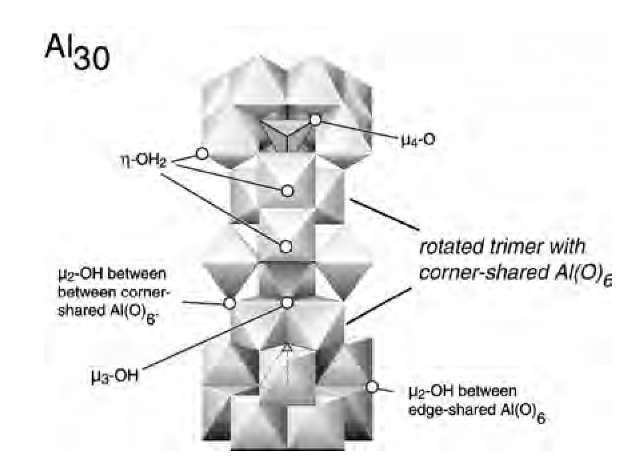
How do nanocrystals form from solution? The classical picture of nucleation and growth by addition of single atoms or ions is probably inadequate.1-14-1 There is increasing evidence for clusters of atoms or ions in solution which contain 5-50 atoms and clearly show some of the structural features of the solid. An example is the Keggin-molecular cluster containing 13 aluminum atoms shown in Fig. 4.[16] It appears stable over a wide range of neutral to basic pH, and is probably a major precursor to and a dissolution product of aluminum oxyhydroxides.[17] The growth of TiO2 anatase may occur by the oriented attachment of ~ 3-nm particles.[18] The growth of zeolites templated by organics may involve 3-nm cuboctahedral clusters.[19] Nanoclusters have been invoked in the growth of sulfides in ore-forming solutions.[20] Characterization of such nanoscale precursors in aqueous solutions remains a major challenge.
The coarsening and phase transformation of nanoscale precipitates upon heating is equally important to the sol-gel synthesis of ceramics and the geologic compaction and diagenesis of buried sediments. Loss of water, loss of surface area, and phase transformations to the stable bulk polymorph are closely interlinked.[14] A nanoparticle with dimensions below 5 nm probably cannot maintain defects or dislocations; they can migrate to the surface and be annihilated.1-21-1 An aggregate of such single domain na-nocrystals, with disorder and impurities at their surfaces, may be a common morphology in nature. Such aggregates give smaller average particle size from X-ray peak broadening than from gas adsorption [Brunauer-Emmett-Teller (BET)] measurements.
Magnetic nanoparticles cannot hold a direction of magnetization for a long time because of thermal fluc-tuations.[22] The magnetic iron oxides found in magneto- tactic bacteria, which are single domain particles, neither too large nor too small, provide orientation in the Earth’s magnetic field (see below). On a geologic time scale (millions of years), magnetization of largely nanophase iron oxides provides a record of the variation of the Earth’s magnetic field through time, including periodic reversals of north and south poles. Thus the ability or inability of nanoparticle oxides to retain magnetization is of critical importance.
Fig. 4 Al2O8Al28(OH)56(H2O)2418+(aq) (often called A^) cluster of 2 nm dimensions, which is intermediate in structure and properties between isolated ions and solid aluminum. (W.H. Casey, personal communications.
NANOPARTICLES IN THE ATMOSPHERE
Atmospheric particles include dust (rock and soil), sea salt, acids including sulfuric, organics (especially carbon), inorganics, and, of course, water and ice (Table 3). The atmosphere can carry particles of spherical equivalent diameters from 1 to 105 nm. Often, a trimodal distribution of particle sizes, with peaks in number density near 5, 50, and 300 nm, is seen.[23] The smaller particles account for most of the reactive surface area but little of the total mass.
Atmospheric particles affect the environment in many ways.[23,24] They reduce visibility (smog, haze) primarily through light scattering. They act as nuclei for water vapor condensation and cloud formation. They are involved in radiative forcing, changing the ratio of absorbed sunlight to reflected sunlight. Thus they are implicated in global climate change. Their effect on radiative forcing can be positive (more energy adsorbed) or negative (more energy reflected), leading to warming or cooling, respectively.[23,24] Their net effect is a subject of vigorous research and controversy.
Table 3 Atmospheric nanoparticles
Liquid droplets Water
Sulfuric acid Nitric acid
Sea water and other salt solutions Organics Solid particles Ice (H2O) NaCl Na2SO4 CaSO4- 2H2O NaNO3 H2SO44H2O HNO3-3H2O
C (graphite, amorphous, fullerenes, nanotubes) SiO2
Iron oxides
Clays
Organics
Many particles have core-shell structures and coatings.
Anthropogenic particles contribute disproportionately to the fine particle fractions.[23] These may have very significant effects on climate and (see below) health. Soot (carbon) from burning coal and oil and from automobile (especially diesel) emissions contributes greatly to the atmospheric load of nanoparticles.
Particles in the atmosphere travel a long way. Dust from Africa is seen in Florida; industrial emissions from China are detected in North America. Particles are removed from the atmosphere by diffusion and gravitational settling (aided by small particles coalescing into larger ones) and by rain. The residence time of nanopar-ticles in the atmosphere ranges from minutes to days.[23]
Atmospheric nanoparticles are more involved in gas phase reactions than particles in soil and water.[24] Their formation may involve combustion synthesis, as in industrial or automobile emission. Mineral nanoparticle surfaces may catalyze the oxidation of SO2 and NO2, leading to sulfuric and nitric acid. These acids can exist as gaseous species, liquids, or solid hydrates at low temperature. Nanoparticles are invoked in the depletion of atmospheric ozone by catalytic production of reactive chlorine compounds. Changes of phase (liquid to solid) are critical to the chemistry of sodium chloride and sodium nitrate particles, with their water content being controlled by available humidity.
Mineral dust particles may provide critical nutrients (e.g., iron) to the surface of the ocean far from land. The ocean’s biological productivity is often limited by the availability of these nutrients; thus such inorganic nano-particles may significantly influence the global cycling of carbon through ocean biomass.[25]
NANOPARTICLES IN SEDIMENTS, ROCKS, AND THE DEEP EARTH
The debris of rock weathering is brought down river to the ocean in sediments consisting of nanoscale particles of clay, small quartz grains, and other minerals. Indeed, the terms ”clay” and ”silt” have a classic connotation of size fraction, although the former also implies a structural group of minerals, the layered aluminosilicates. In the ocean, carbonates precipitate, dissolve, and reprecipitate as a complex function of depth.[26] Both silica and various polymorphs of calcium carbonate (calcite, aragonite, vaterite) are produced by organisms such as diatoms, foraminifera, and corals. Their debris rains down on the ocean bottom, forming sediments which often show annual cycles in composition and texture and which bear records of climate change, shifts in ecosystems, and catastrophic events such as meteor impacts.[27] These sediments start off largely nanoscale. They coarsen and dehydrate with time and depth of burial. The evolution of their organic matter leads to petroleum. The evolution of their minerals, involving coarsening and compaction, called diagenesis, leads to rocks such as limestones and shales. The nanoscale processes that take place (dehydration and organic loss, phase transformation, coarsening and densification) are natural analogs of ceramic processing which starts with nanoscale precipitates or gels.
Natural processes involving changes in temperature, pressure, acidity, and oxygen fugacity cause the concentration of trace metals into ore deposits. These often occur in hydrothermal systems, spatially contained circulations of hot, pressurized, metal-rich aqueous solutions. Our ability to mine low-grade deposits by chemical leaching techniques brings us into the world of nanoparticles and reactions at mineral surfaces. There is increasing evidence that microorganisms play an active role in ore deposi-tion.[28,29] Hot springs at the surface produce deposits of nanoscale amorphous silica and other minerals, which may also be closely linked to microbial activity.[30]
At temperatures above a few hundred degrees Centigrade and pressures above a few kilobars, coarse-grained metamorphic and igneous rocks predominate. The interior of the Earth is layered, with seismic discontinuities delineating the crust, upper mantle, transition zone, lower mantle, and core. These discontinuities represent regions of rapidly changing density, mineralogy, and chemistry.[31] Ongoing phase transitions and chemical reactions can decrease the grain size of a material and render it easier to deform.[32] Thus nanoscale phenomena, occurring at specific locations, may play a disproportionate role in processes such as subduction, plate tectonics, earthquake generation, and volcanism. Shock processes, (e.g., meteor impact, nuclear detonation) also produce nanoparticles.
When a volcano erupts explosively, a plume of dust particle is sent into the atmosphere, sometimes reaching the stratosphere. These particles make beautiful sunsets but they also exert a significant cooling effect on climate for several years and pose a significant aviation hazard. Combining sedimentation, coarsening, subduction, volcan-ism, and weathering, there is an ongoing global geo-chemical cycle of nanoparticles, analogous in some ways to global geochemical cycles of elements such as carbon. However, the mass balances, or imbalances, in global nanoparticle production and consumption through time have not been characterized.
NANOPARTICLES BEYOND THE EARTH
In the early stages of planet formation, dilute and more or less uniform gas condensed to form a series of mineral particles, with the order of condensation described by thermodynamic calculations based on the volatility and stability of these phases.[37] The more refractory oxides condensed earlier than those with higher volatility. These particles accreted, under the influence of gravity, to form our solar system. What was the nature of these initial particles? What was their size distribution? Were they crystalline or amorphous? Were metastable polymorphs formed? While the initial high temperatures might argue against such metastability, the low pressures and condensation from a vapor argue for it. In technological processes, chemical vapor deposition produces nanoscale amorphous silica ”snow,” and combustion produces soot and inorganic nanoparticles. The role of nanoparticles in planetary accretion has not yet been explored. The change in stability at the nanoscale, which will be different for various compositions and polymorphs, may alter the sequence of condensation of phases. Are the particles now present in space as interplanetary dust partly or mostly nanoparticles?
The surfaces of the Moon and Mars, subject to ”space weathering” by bombardment with meteorites of all sizes, contain an extensive fine grained dust or soil layer.[32] Samples of lunar soil, brought back by the Apollo missions, contain a distribution of particle sizes of spherules and irregular shards. Their particle size distribution appears not to have been a subject of active interest, but clearly a significant number are in the nanoregime. The red surface of Mars appears to be dominated by various fine-grained or nanophase iron oxides. Until Martian sample return missions, planned to occur in the next decade or two, bring some of this material to Earth, we must rely on remote sensing technology (spectroscop-ic techniques) and instrumentation on Martian landers (possibly Mossbauer spectroscopy, X-ray fluorescence, and X-ray diffraction) to obtain information on the composition and structure of Martian soil. Considering the difficulty of characterizing iron oxide nanoparticles in the best laboratories on Earth, definitive conclusions about the nature of Martian soil are unlikely until we have some samples in hand. Meteorites believed to be from Mars contain micron-sized spherules, which were proposed to be biological in origin. This sparked much recent controversy and it is by no means settled whether these structures are fossil microorganisms or the product of inorganic nanoscale crystal growth processes.[33,34]
NANOPARTICLES AND LIFE
Microbial communities are rich in the production and utilization of nanoparticles.[35] Table 4 lists some examples. In addition to aerobic respiration (the enzymatic oxidation of carbohydrates and other organics with molecular oxygen to produce water, carbon dioxide, and energy stored as high-energy phosphate linkages) organisms use many other strategies to extract energy from the environment. The following biological reactions produce or consume nanoparticles. Dissolved Mn(II) or Fe(II) can be oxidized by oxygen, producing Mn(III), Mn(IV), or Fe(III) oxide nanoparticles, while organics can be oxidized by manganese or iron oxides, producing soluble Mn(II) and Fe(II) species. Some bacteria can also utilize the U(IV)-U(VI) couple as an energy source. Because hexavalent uranium is much more soluble than tetravalent, biological processes that accelerate its production are of concern in modeling nuclear waste leaching. The sum of these two groups of redox processes is the oxidation of organics by oxygen, akin to respiration. The important difference is that the organic food source and the oxygen source can be spatially separated in the sharp gradients in oxygen and organic contents that frequently occur in sediments, and different communities of organisms participate in the two processes. In marine sediments, sulfate is the dominant biological electron acceptor and is more important than oxygen. Bacterial sulfate reduction produces sulfide which often precipitates as nanophase metal sulfide minerals. Sulfide and sulfur oxidizing bacteria typically live in specialized environments where there is enough oxygen to oxidize sulfur but not so much that chemical oxidation swamps biological oxidation. This oxidation consumes solid sulfur and sulfides, and produces soluble sulfate.
Table 4 Example of interactions of microorganisms and nanoparticles
|
Class of organisms |
Example |
Nanoparticle interaction |
|
Iron and manganese |
Thiobacillus |
Oxidize soluble Mn2+ and Fe2+ to insoluble |
|
oxidizing bacteria |
higher oxides |
|
|
Iron and manganese |
Shewenella |
Reduce insoluble Mn and Fe oxides to soluble forms |
|
reducing bacteria |
oxidize sulfide to sulfate to sulfide precipitate |
|
|
Sulfur reducing bacteria |
Thiobacillus |
Reduce sulfate to sulfur in sulfide |
|
Magnetotactic bacteria |
Aquaspirillum |
Nanoparticles of Fe2O3, Fe3O4, and/or iron sulfides. |
|
magnetotacticum |
Soluble U6+! insoluble U4 + |
|
|
Uranium reducing bacteria |
Geobacter, shewenella |
|
|
Fungi |
Specific strains unknown |
Oxidize Mn2+, precipitate MnO2 |
|
Diatoms |
Various |
Precipitate silica |
|
Foraminifera |
Various |
Precipitate CaCO3 calcite and aragonite |
Organisms utilize nanoparticles in processes other than respiration. Bacterial precipitation of sulfide minerals, e.g., ZnS and UO2, may also be a mechanism of detoxification.1-36-1 Similar detox processes may occur in plants. Magnetotactic bacteria synthesize and align single domain magnetic iron oxide and iron sulfide particles in structures called magnetosomes.[37] Such bacteria align themselves both north-south and vertically in the Earth’s magnetic field. The navigational (homing) capabilities of bees, pigeons, and probably other higher organisms utilize magnetic field orientation sensed by magnetic iron oxide particles in their brains. Similar particles, although at lower abundance, occur in many mammals, including Homo sapiens.[38] There has been a debate in the public sector whether the magnetic fields produced by high-voltage power lines are potentially dangerous to human health. In contrast, the use of magnets in alternative medicine, and the market for magnetic pillows, back supports, etc. suggests, or at least hopes for, a beneficial effect of the interaction of magnetic fields with animals. Key to either harmful or helpful biological effects is a mechanism for the magnetic field to interact with living cells. Interaction with biological magnetic nanoparticles may provide such a mechanism, but very little is known at present.
Nanoparticles have other documented health effects.[39] When inhaled into the lungs, particles cause an inflammatory response, which contributes to allergies, asthma, and cancer.[48] The detailed mechanism of this response, and how it depends on surface area, particle size, or specific particle chemistry, is not clear. The harmful effects of inhaled particles may be enhanced by other pollutants, particularly ozone, typically present in smog. Nanoparticles penetrate deep into the lungs. Many are returned with exhaled air, some stick to the surfaces of the alveoli, and some may even penetrate into general blood circulation and be transported to other organs. Studies linking detailed nanoparticle characterization, biochemical and physiological processes, and health effects are just beginning to be carried out. It is likely that not all particles have comparable effects, and understanding which are the most dangerous could lead to rational, rather than arbitrary, emission standards for automotive and industrial particulates.
In the early Earth, prebiotic processes culminated in the origin of life.[40] Because the synthesis of complex organic molecules competes with their destruction by hydrolysis and other degradation, it is possible that the most successful synthesis could have occurred in sheltered and catalytic environments, such as those provided by mineral surfaces, nanoparticle surfaces, and pores within mineral grains. Present-day organisms utilize a wide variety of elements (e.g., Fe, Co, Ni, Cr, Zn, Se) in specific enzymes. Although large amounts of such elements are toxic, trace amounts are essential. The active centers in enzymes utilizing these trace elements often consist of clusters of metal atoms, sometimes associated with sulfide. Are these fine-tuned by evolution from earlier simpler metal clusters and nanoparticles existing in the environment? Thus nanoparticles may play a role not just in the sustenance of life but in its origin.
CONCLUSION
Nanoparticles play diverse roles in the environment and are involved in both abiotic and biologically mediated chemical and physical processes. Their high surface area, chemical reactivity, polymorphism, and unique properties involve nanoparticles in a disproportionately large fraction of the chemical reactions occurring on and in the Earth and other planets. Understanding this involvement is itself evolving into a new field of study in the environmental and Earth sciences, which is beginning to be called ”nanogeoscience.” Nanogeoscience will take its place alongside other new areas such as astrobiology and bio-geochemitry, fields that link physical, chemical, and biological processes viewed in the context of the long time and distance scales natural to the Geosciences.
![(a) Enthalpies of alumina polymorphs as a function of surface area. (From Ref. [3].) (b) Enthalpies of titania polymorphs as a function of surface area. The heavy lines show the stable polymorphs in each size range. (a) Enthalpies of alumina polymorphs as a function of surface area. (From Ref. [3].) (b) Enthalpies of titania polymorphs as a function of surface area. The heavy lines show the stable polymorphs in each size range.](http://what-when-how.com/wp-content/uploads/2011/03/tmp1C167_thumb_thumb.jpg)