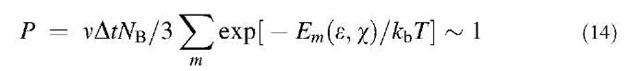FAILURE AND RELAXATION MECHANISMS
The important issue of ultimate tensile strength of CNTs is inherently related with the atomic relaxation in the lattice under high strain. This thermally activated process was first predicted to consist of a sequence of individual bond rotations in the approach based on dislocation theory.[22,25,26] Careful computer simulations demonstrate feasibility of this mechanism and allowed us to quantify important energy aspects.[27,28] It has been shown that in a crystal lattice such as the wall of a CNT, a yield to deformation must begin with a homogeneous nucleation of a slip by the shear stress present. The nonbasal edge dislocations emerging in such slip have a well-defined core, a pentagon-heptagon pair, 5/7. Therefore the prime dipole is equivalent to the Stone-Wales (SW) defect. The nucleation of this prime dislocation dipole ”unlocks” the nanotube for further relaxation: either brittle cleavage or a plastic flow. Remarkably, the latter corresponds to a motion of dislocations along the helical paths (glide ”planes”) within the nanotube wall. This causes a stepwise (quantized) necking, when the domains of different chiral symmetry and therefore different electronic structure are formed, thus coupling the mechanical and electrical properties.[22,25,26] It has further been shown[22,23,25,28-30] that the energy of such nucleation explicitly depends on CNT helicity (chirality).
Below, we deduce starting with dislocation theory the atomistics of mechanical relaxation under extreme tension. Locally, the wall of a nanotube differs little from a single graphene sheet, a 2-D crystal of carbon. When a uniaxial tension s (N/m—for the 2-D wall, it is convenient to use force per unit length of its circumference) is applied, it can be represented as a sum of expansion (locally isotropic within the wall) and a shear of a magnitude s/2 (directed at ±45° with respect to tension). Generally, in a macroscopic crystal, the shear stress relaxes by a movement of dislocations, the edges of the atomic extraplanes. Burgers vector b quantifies the mismatch in the lattice as a result of a dislocation. Its glide requires only local atomic rearrangements and presents the easiest way for strain release, provided sufficient thermal agitation. In an initially perfect lattice such as the wall of a nanotube, a yield to a great axial tension begins with a homogeneous nu-cleation of a slip, when a dipole of dislocations [a tiny loop in three-dimensional (3-D) case] first has to form. The formation and further glide are driven by the reduction of the applied-stress energy, as characterized by the elastic Peach-Koehler force on a dislocation. The force component along b is proportional to the shear in this direction and thus depends on the angle between the Burgers vector and the circumference of the tube,
The max f,| is attained on two ±45° lines, which mark the directions of a slip in an isotropic material under tension.
The graphene wall of the nanotube is not isotropic; its hexagonal symmetry governs the three glide planes—the three lines of closest zigzag atomic packing, oriented at 120° to each other (corresponding to the {101 l} set of planes in 3-D graphite). At nonzero shear, these directions are prone to slip. The corresponding c-axis edge dislocations involved in such slip are indeed known in graphite. The six possible Burgers vectors 1/3a (2110) have a magnitude b=a= 0.246 nm (lattice constant), and the dislocation core is identified as a 5/7 pentagon-heptagon pair in the honeycomb lattice of hexagons. Therefore the primary nucleated dipole must have a 5/7/7/5 configuration (a 5/7 attached to an inverted 7/5 core). This configuration is obtained in the perfect lattice (or a nanotube wall) by a 90° rotation of a single C—C bond, well known in fullerene science as a Stone-Wales diatomic interchange. One is led to conclude that the SW transformation is equivalent to the smallest slip in a hexagonal lattice and must play a key role in the nanotube relaxation under external force.
The preferred glide is the closest to the maximum shear ±45° lines and depends on how the graphene strip is rolled up into a cylinder. This depends on nanotube helicity specified by the chiral indices (c1, c2) or a chiral angle 0 indicating how far the circumference departs from the leading zigzag motif ai. The max fb| is attained for the dislocations with b=±(0,1) and their glide reduces the strain energy
per one displacement, a. Here e is the applied strain and C = Yh=342 N/m can be derived from the Young’s modulus of Y =1020 GPa and the interlayer spacing h=0.335 nm in graphite; one then obtains Ca2/2=64.5 eV. Eq. 11 allows one to compare different CNTs (assuming similar amount of preexisting dislocations): the more energetically favorable is the glide in a tube, the earlier it must yield to applied strain.
In a pristine nanotube molecule, the 5/7 dislocations have to first emerge as a dipole by a prime SW transformation. Topologically, the SW defect is equivalent to either one of the two dipoles, each formed by an — a/2 slip. Applying Eq. 11 to each of the slips, one finds
The first two terms, the zero-strain formation energy and possible isotropic dilation, do not depend on chiral symmetry. The symmetry-dependent third term, which can also be derived as a leading term in the Fourier series, describes the fact that SW rotation gains more energy in an armchair (0=30°) CNT, making it thermodynamically the weakest and most inclined to SW nucleation of the dislocations, in contrast to the zigzag (0=0) where the nucleation is least favorable.
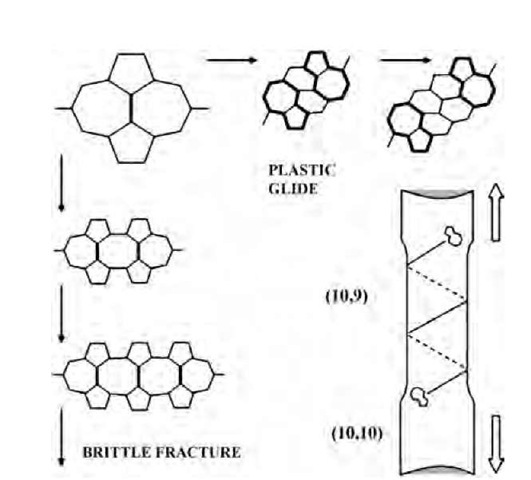
Fig. 7 SW transformations of an equatorially oriented bond into a vertical position create a nucleus of relaxation (top left corner). It evolves further as either a crack—brittle fracture route, left column—or as a couple of dislocations gliding away along the spiral slip ”plane” (plastic yield, top row). In both cases, only SW rotations are required as elementary steps. The stepwise change of the nanotube diameter reflects the change of chirality, bottom right image, causing the corresponding variations of electrical properties.
Consider for example a (c,c) armchair CNT as a typical representative (we will also see below that this armchair type can undergo a more general scenario of relaxation). The initial stress-induced SW rotation creates a geometry that can be viewed as either a dislocation dipole or a tiny crack along the equator. Once ”unlocked,” the SW defect can ease further relaxation. At this stage, both brittle (dislocation pileup and crack extension) and plastic (separation of dislocations and their glide away from each other) routes are possible, the former usually at larger stress and the latter at higher temperatures.
Formally, both routes correspond to a further sequence of SW switches. The 90° rotation of the bonds at the ”crack tip” (Fig. 7, left column) will result in a 7/8/7 flaw and then 7/8/8/7, etc. This further strains the bonds— partitions between the larger polygons, leading eventually to their breakage, with the formation of greater openings such as 7/14/7, etc. If the crack, represented by this sequence, surpasses the critical Griffith size, it cleaves the tubule.
In a more interesting distinct alternative, the SW rotation of another bond (Fig. 7, top row) divides the 5/7 and 7/ 5, as they become two dislocation cores separated by a single row of hexagons. A next similar SW switch results in a double-row separated pair of the 5/7′s and so on. This corresponds, at very high temperatures, to a plastic flow inside the nanotube molecule, when the 5/7 and 7/5 twins glide away from each other driven by the elastic forces, thus reducing the total strain energy (cf. Eq. 11). One remarkable feature of such glide is a result of mere cylindrical geometry: the glide ”planes” in case of nanotubes are actually spirals, and the slow thermally activated Brownian walk of the dislocations proceeds along these well-defined trajectories. Similarly, their extraplanes are just the rows of atoms also curved into the helices.
A nanotube with a 5/7 defect in its wall loses axial symmetry and has a bent equilibrium shape; the calculations show[31] the junction angles < 15°. Interestingly then, an exposure of an even achiral nanotube to the axially symmetric tension generates two 5/7 dislocations, and when the tension is removed, the tube ”freezes” in an asymmetric configuration, S-shaped or C-shaped, depending on the distance of glide, i.e., time of exposure. This seemingly ”symmetry-violating” mechanical test is a truly nanoscale phenomenon. Of course, the symmetry is conserved statistically because many different shapes form under identical conditions.
When the dislocations sweep a noticeable distance, they leave behind a tube segment changed strictly following the topological rules of dislocation theory. By considering a planar development of the tube segment containing a 5/7, for the new chirality vector c’, one finds
with the corresponding reduction of diameter, d. While the dislocations of the first dipole glide away, a generation of another dipole results, in further narrowing and proportional elongation under stress, thus forming a neck as shown above. The orientation of a generated dislocation dipole is determined every time by the Burgers vector closest to the lines of maximum shear (±45° cross at the endpoint of the current circumference vector c). The evolution of a (c,c) tube will be: (c,c)! (c,c — 1)! (c,c — 2)!.. .(c,0)! [(c — 1,1) or (c, — 1)]! (c — 1,0)! [(c — 2,1) or (c — 1, — 1)]! (c — 2,0)! [(c — 3,1) or (c — 2, — 1)]! (c — 3,0), etc. It abandons the armchair (c,c) type entirely, but then oscillates in the vicinity of zigzag (c,0) kind, which appears a peculiar attractor. Correspondingly, the diameter for a (10,10) tube changes stepwise, d =1.36, 1.29, 1.22, 1.16 nm, etc., the local stress grows in proportion, and this quantized necking can be terminated by a cleave at late stages. Interestingly, such plastic flow is accompanied by the change of electronic structure of the emerging domains, governed by the vector (c1,c2). The armchair tubes are metallic, and others are semiconducting with the different band gap values. The 5/7 pair separating two domains of different chirality has been discussed as a pure-carbon heterojunction, is argued to cause the current rectification detected in a nanotube nanodevice,[32] and can be used to modify, in a controlled way, the electronic structure of the tube. Here we see how this electronic heterogeneity can arise from a mechanical relaxation at high temperature: if the initial tube was armchair-metallic, the plastic dilation transforms it into a semiconducting type irreversibly.[25,26,33]
While the above analysis is based on atomic picture (structure and interactions), recent developments[34] offer an approach where the fracture nucleation can be described rather elegantly within nonlinear continuum mechanics (based on classical interatomic forces for carbon). Whether this approach can describe change in chirality, temperature dependence, or temporal aspects of relaxation should yet be explored.
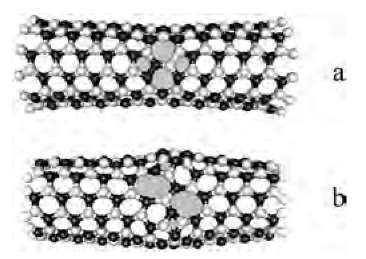
Fig. 8 The geometries of (5,5) BN tubule with (a) 5/7/7/5 defect emerging at high tension and temperature and (b) 4/8/8/4 dislocation dipole.
Fig. 9 T =3000 K, strain 3%, plastic flow behavior (about 2.5 nsec). The shaded area indicates the migration path of the 5/7 edge dislocation.
The dislocation theory allows one to expand the approach to other materials, and we have recently applied it to boron nitride (BN) nanotubes.[35,36] While the binding energy in BN is lower than in CNT, the formation of 5/7/ 7/5 defect can be more costly because of Coulomb repulsion between emerging BB and NN pairs (Fig. 8a) (bonding in BN is partially ionic with strong preference to chemical neighbor alternation in the lattice). Another dislocation pair 4/8/8/4 that preserves the alternation must be considered (Fig. 8b). It turns out that the quantitative results are sensitive to the level of theory accuracy. Tight binding approximation[37] underestimates the repulsion by almost 3 eV.[35,36] Ab initio DFT calculations show that 5/7/7/5 is metastable lowest energy defect in BN, and its formation energy 5.5 eV is higher than 3.0 eV in carbon,[35,36] thus suggesting higher thermodynamic stability of BN under tensile load. Relaxation under compression is different as it involves skin-type buckling also investigated recently.[38]
KINETIC APPROACH TO STRENGTH-FAILURE EVALUATION
Computer simulations have provided a compelling evidence of the mechanisms discussed above. By carefully tuning a quasi-static tension in the tubule and gradually elevating its temperature, with extensive periods of MD annealing, the first stages of the mechanical yield of CNT have been observed (Fig. 9).[27,28] In simulation of tensile load, the novel patterns in plasticity and breakage, just as described above, clearly emerge. At very high strain rate, the details of primary defects cannot be seen and they only begin to emerge at higher strain level, giving impression of exceedingly high breaking strain.[17]
Fracture, of course, is a kinetic process where time is an important parameter. Even a small tension, as any non-hydrostatic stress, makes material thermodynamically metastable and a generation of defects energetically favorable. Thus the important issue of strength remains beyond the defect formation energy and its reduction with the applied tension. Recently, we developed kinetic theory in application to CNT.[30,39] In this approach, we evaluate conditions (strain e, temperature T) when the probability P of defect formation becomes significant within laboratory test time At,
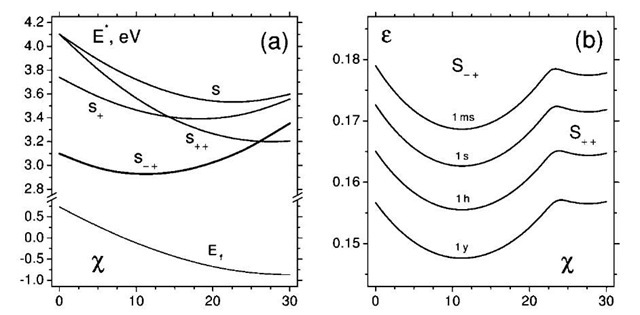
Fig. 10 Activation barrier values (here computed within classical multibody potential, a) serve as input to the rate equation (14) and the calculation of the yield strain as a function of time (here from 1 msec to 1 year, b), temperature (here 300 K), and chiral symmetry (W).
Here v = kbT/h is the usual attempt frequency and NB is the number of bonds in the sample. Activation barrier Em(e,w) must be computed as a function of strain and chirality w of the tubule, and then the solution of this equation with respect to e gives the breaking strain values. This approach involved substantial computational work in finding the saddle points and energies (Fig. 10a) for a variety of conditions and for several transition state modes (index m in the summation above). Obtained yield strain near 17% (Fig. 10b,[3a39]) is in reasonable agreement with the series of experimental reports. We currently are implementing similar approach with ab initio level of saddle point barrier calculations.[40] Preliminary data show higher 8-9 eV barriers, but their reduction with tension is also faster.
Previously performed high strain rate simulations have shown temperature dependence of breaking strain,[14,17] consistent with the kinetic theory.[30] In a constant strain rate arrangement (when the ends of the sample are pulled from the equilibrium with certain rate), the rate equation is slightly modified to its integral form. However, the main contribution comes from the vicinity of the upper limit,
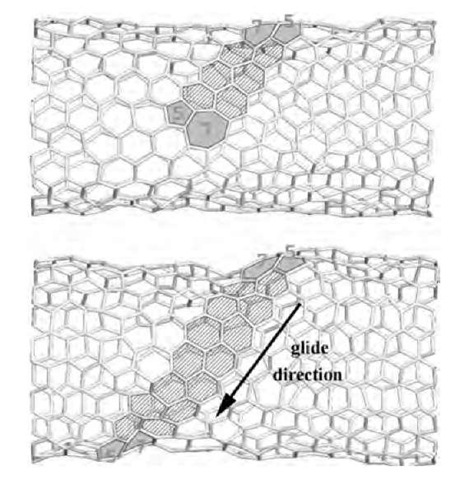
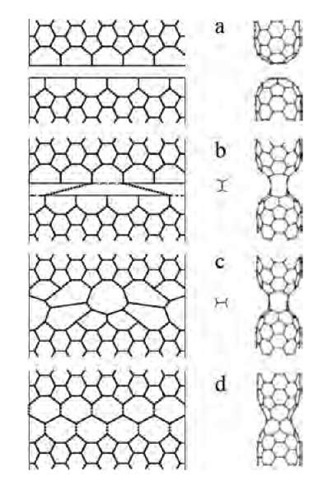
Fig. 11 2-D geodesic projection (left) and the actual 3-D structures (right) show the transformations from a pair of separate (5,5) tubes (a) to a single defect-free nanotube. Primary ”polymerization” links form as two other bonds break (b, dashed lines). The p/2 rotations of the links (the bonds subject to such SW flip are dotted) and the SW flips of the four other bonds in (c) produce a (5,0) neck (d). It widens by means of another 10 SW rotations, forming a perfect single (5,5) tubule (not shown).
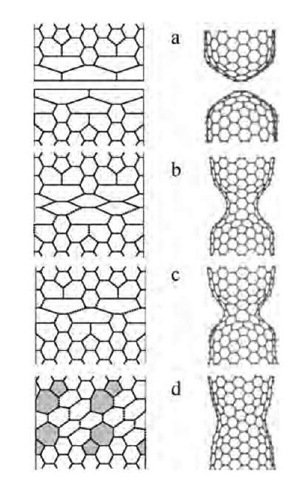
Fig. 12 2-D projections (left) and the computed 3-D intermediate structures (right) in the coalescence of the two (10,10) nanotubes: separate caps (a) in a sequence similar to Fig. 8 develop a (5,5) junction (b), which then shortens (c) and widens into a (10,5) neck (d). Glide of the shaded 5/7 dislocations completes the annealing into a perfect (10,10) CNT (not shown). Due to the fifth-fold symmetry, only two cells are displayed.
Simple analysis reveals certain invariant Tx log(vAt) of the time of failure and temperature (provided the constant strain). Detailed simulations could shed additional light on this aspect (Cho et al., private communication, 2002). More significant finding reported recently[41] is that kinetic probability of SW bond rotation at room temperatures can be rather small and the actual mechanism of failure is a brittle cleave, through a sequence of ”lattice-trapped” states corresponding to individual broken bonds, from one to two, three, etc.[41]
COALESCENCE OF NANOTUBES AS A REVERSED FAILURE
Understanding the details of failure mechanism has led us recently[42-44] to investigate an opposite process, a coalescence of nanoscale clusters analogous to macroscopic sintering or welding. Fusion of smaller components into a larger whole is a ubiquitous process in condensed matter. In molecular scale, it corresponds to chemical synthesis, where exact rearrangement of atoms can be recognized. Coalescence or sintering of macroscopic parts is usually driven by the well-defined thermodynamic forces (frequently, surface energy reduction), but the atomic evolution path is redundant and its exact identification is irrelevant. Exploring a possibility of the two particles merging with atomic precision becomes compelling in nanometer scale, where one aspires to ”arrange the atoms one by one.” Are the initial and final states connected by a feasible path of atomic movements, or separated by insurmountable barriers? Direct molecular dynamics (MD) investigation is usually hampered by energy landscape traps and beyond very few atomic steps needs to be augmented with additional analysis.
An example of very small particles is offered by ful-lerene cages and CNTs. Fusion of fullerenes has been previously reported and the lateral merging (diameter-doubling) of CNT has been observed and simulated.[45,46] In contrast, head-to-head coalescence of CNT segments remained unexplored and of particular theoretical and practical interest: Is it permitted by rigorous topology rules to eliminate all the pentagons always present in the CNT ends and thus dissolve the caps completely? Can this occur through a series of well-defined elementary steps and what is overall energy change if the system evolves through the intermediate disordered states to the final purely hexagonal lattice of continuous tubule? If feasible, such ”welding” can lead to increase of connectivity in CNT arrays in bundles/ropes and crystals and thus significantly improve the mechanical, thermal, and electrical properties of material. In addition, determining the atomistic steps of small-diameter tube coalescence (with the end caps identical to half-buckyball) can shed light on the underlying mechanism of condensed phase conversion or CNT synthesis from C60 components.
In Ref. [42] we have reported for the first time atomically precise routes for complete coalescence of generic fullerene cages: cap-to-cap CNT and C60 merging together to form defectless final structure. The entire process is reduced to sequence of Stone-Wales bond switches and therefore is likely the lowest energy path of transformation. Several other examples of merging follow immediately as special cases: coalescence of buckyballs in peapod, joining of the two (5,5) tubes as in Fig. 11, ”welding” the (10,10) to (10,10) following Fig. 12, etc. The approach remains valid for arbitrary tubes with the important constraint of unavoidable grain boundary for the tubes of different chirality. The junction of (n,m) and (n’,m’) must contain 5/7 dislocations or their equivalent of (n — n’,m — m’) total Burgers vector.[25] The proposed mechanism[42-44] has important implications for nano-tube material (crystals, ropes) processing and property enhancement, engineering of nanoscale junctions of various types, possible growth mechanisms with the C60 and other nanoparticles as feedstock. In the context of nano-mechanics, an interesting feature of the late stages of coalescence is the annealing and annihilation of 5/7 pairs in a process exactly reverse to the formation and glide of these dislocation cores in the course of yield and failure under tension. On the other hand, although covalent bond flip represents intramolecular process, it is remarkable how it can lead to possibility of global supramolecular shape changes and reactions, from merging coalescence to interpenetration and encapsulation.
Fig. 13 Molecular mechanics (relaxed with realistic interatomic force-field and weak van der Walls interaction) simulation of the simplest tensegrity structure c3t9 of carbon nanotube beams connected by covalently attached to the cap pentagons polyethylene chains.
TENSEGRITY AT SUPRAMOLECULAR SCALE
Usual supramolecular interactions possess the same generic properties as any other interatomic forces: weak but always present attraction at large distances (from tens of nanometers) results in a relatively shallow potential minimum at intermolecular vicinity of 0.2-0.5 nm spacing. Closer, it turns into a steep repulsive potential which, upon sufficient compression, can yield to a covalent bonding. Therefore supramolecular forces by themselves are unlikely to be able to support low-density assembly of molecules and nanotubes in particular: they would form aligned bundles or at least relatively dense random mats. In this context, it became of interest recently to explore transferability of certain ideas of macroengineering and to connect rigid nanotubes by chemically attached polymer segments to keep them apart. This seems at a first glance counterintuitive—how adding flexible links can prevent rigid beams from lumping together? However, at the macroscopic scale, the idea goes back to Fuller,[47] who has proposed and patented tensile-integrity structures, hence the terms tensegrity in the literature. Tensegrity is a design principle that describes how network structures achieve shape stability. The main point is that a disconnected set of rigid beams can be tethered by sparse series of tensile threads (each perfectly bendable and unsup-portive to any compression) in such a way that global structure can become rigid, sustain compressive global load, and possess all-finite vibration eigenfrequencies. Such engineered structures can be found in architecture, furniture, entertainment, and aerospace applications (because of lightweight and ”foldability”). In cell biology, cytoskeleton is also suggested to possess tensegrity properties.[48] Fig. 13 shows simplest simulated tensegrity unit (3 beams compression members connected by 9 chains tension members, c3t9 per Skelton’s nomenclature) composed of rigid carbon nanotubes tethered by polymer chains.[49] We have analyzed stability changes in such and several similar structures upon removal of one chain or adding a few extras.
CONCLUSION
Mechanics of nanotubes has attracted keen interest since their discovery and over the past decade. Above, we have described several established facts and properties together with other, yet speculative aspects and possibilities. It may be useful to reiterate in conclusion some observations and notions of the field as discussed in this article.
Because of qualitative differences, nanotube mechanics can be subdivided into intramolecular, involving bond rearrangements and atomic removal or insertion within the carbon lattice of the tube wall, and supramolecular mechanics of interactions between the tube molecules. As illustrated above, the latter can be a result of weak distant interactions via dispersion van der Waals forces, stronger direct electrostatic potentials, or by means of much stronger covalent linkage-tethering of nanotubes through intermediate functional groups or elongated chains (as in the example of tensegrity designs). While supramolecular interactions play central role in colloid chemistry, in numerous biochemical processes, and have been broadly studied, in case of nanotubes, certain special aspects stand out. Great strength of carbon lattice makes it qualitatively different from most other molecules often involved in supramolecular processes: lowest activation barrier for intratube bond rearrangements is 7-9 eV high, which makes even covalent bonding with some other species or between the tubes relatively small supramo-lecular pertubation.
In contrast, the very same stiffness and ”surface” smoothness of the tubes results in dramatic amplification or the traditionally weak dispersion forces. Van der Waals interactions add up along the tube-tube contact and yield typically eV per each nanometer of length, i.e., many electron volts for a typical lateral contact. In turn, this makes solubility or even mechanical separation of nano-tube bundles a very challenging task. These distinct features of supramolecular mechanics of nanotubes play critical role in their assembly in arrays-bundles of ropes, nematic crystals, or in their distribution in and coupling with solvents or matrix substances in composite materials.