INTRODUCTION
A great deal of interest has been focused on nanomaterials in the last decade because of their unusual physical, mechanical, and chemical properties. They have found numerous applications, or are expected to find such applications in electronics, catalysis, composite materials, etc. The differences in their chemical properties, in particular, compared with the corresponding bulk material are mostly because of a largely increased surface area and a great increase in the number of active sites on the surface, such as corners, edges, and dislocations. Furthermore, nanomaterials are usually less thermodynamically stable than the corresponding bulk materials, which contributes to their enhanced chemical activity.
Decontamination of chemical and biological warfare is of considerable interest not only for eliminating the hazard of warfare agents on a battlefield, but also in cases of terrorist attacks, industrial accidents, demilitarization of warfare stockpiles, etc. The application of solid materials as decontaminants for both chemical and biological warfare agents has been severely limited by the incomplete and generally slow interaction of solid materials with warfare agents. Nanoparticles with their high surface area, enhanced chemical reactivity, and easy deployment allow the development of new perspectives regarding decontamination. In addition, generally, the products of these decontamination reactions are benign, mineral-like solids.
CHEMICAL WARFARE AGENTS DECONTAMINATION
A component of a filtration system is always a high surface area solid, usually activated carbon, which adsorbs warfare agents physically but does not destroy them. The sorbed material is still highly toxic and the decontamination of the contaminated carbon is a problem. Nanopar-ticles offer surface areas comparable with active carbon, but at the same time high chemical reactivity toward the warfare agents, allowing their chemical decomposition and detoxification.
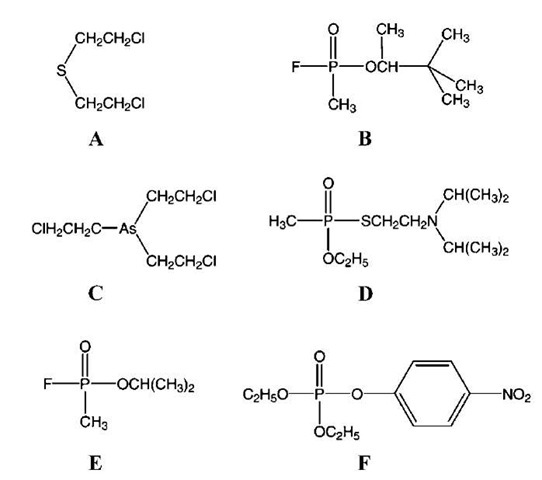
Several chemical warfare agents are noted as most important because of their very high toxicity, persistence, and large existing stockpiles.[1] Mustard gas or HD is a blistering agent, which can be lethal in high concentrations. It irreversibly alkylates key amines in organisms such as proteins, enzymes, and especially DNA, which causes cell malfunction and death. Another warfare agent with similar action and chemical structure is the arsine analog, Lewisite (Fig. 1).
Another large class of chemical warfare agents is the nerve agents class, which is based on phosphonic acid derivatives. Some of them feature a relatively stable bond with fluorine-like sarin (GB agent) or soman (GD agent) (Fig. 1). The later generation, such as VX, contains a more stable ester bond, which contributes to higher persistence, slower hydrolysis, and decontamination, which is more difficult. The VX generation of warfare agents is more toxic as well. The action of the nerve agent is based on its ability to selectively bind to the active centers of key phosphatase enzymes (such as acetylcholinesterases), which are responsible for nerve impulse propagation. Similar to nerve gases are certain widely used pesticides, such as paraoxon (Fig. 1).
Physical Action
There are several requirements for successful decontam-ination,[1] the most important of which is that nontoxic byproducts must be formed and the decontamination should be quick and complete. There is a requirement that the decontamination agent should not be corrosive to the surfaces to which it is applied. Nanoparticles do have excellent properties in this respect, as they are very easy to deploy and to clean up. In general, they are not corrosive, or not as corrosive as typical developed decontamination compositions and solutions. As such, nanoparticulate formulations should be applicable to all kind of surfaces, including the skin, metals, and sensitive equipment, including electronics. Most decontaminating compositions are liquids, which contain organic liquid, electrolytes, as well as corrosive oxidants. Nanoparticles are in the form of fine powder, which can be deployed by an inert solvent suspension such as freon or just by carrier gas under pressure. They can be removed by standard vacuum cleaning procedures. Alternatively, if an air-filtering or water-filtering approach is needed, nanoparticles can be used in the form of porous reactive pellets.
Fig. 1 Structures of the most common chemical warfare agents. (A) 2,2′-Dichloroethyl sulfide (mustard gas or HD); (B) soman or GD; (C) Lewisite; (D) VX agent; (E) sarin or GB; and (F) paraoxon (pesticide).
CHEMICAL ACTION
It has already been mentioned that chemical warfare agents are usually alkylating agents. Usually their hydrolysis renders them nontoxic or much less toxic than warfare agents because the chemical groups that determine toxicity are usually the most reactive parts of the molecule.[1] Similarly, detoxification can be achieved by their oxidation[1-3] because both HD and nerve gases contain relatively easy-to-oxidize sulfur (HD and VX) and/or phosphorus atoms, which have a large contribution to their extreme toxicity.
Metal oxide nanoparticles are apparently a very good choice for detoxification of warfare agents because of the presence of a huge number of basic Lewis and Bronsted sites, as well as acidic sites, which can very significantly accelerate hydrolysis process. By-products, in the form of phosphonates and alcohols, have the tendency to bind strongly to solid surfaces and be retained there. Taking into consideration the high surface area of nanomaterials, this corresponds to a large amount of decomposed warfare agent and rapid reactions compared with bulk metal oxide surfaces because the rate of the interaction is proportional to the contact area. It is additionally strongly facilitated on corners and edges, which have higher activity and lower activation energy barriers compared with normal surface sites.
Most interesting for this type of application are certain metal oxides that have high melting points and chemical stability. The nanomaterial form is thermodynamically less stable than the corresponding bulk material. The higher the melting point is (higher lattice energy), the more stable the nanomaterial will be. Based on this reaction, the best metal oxide nanoparticle systems are magnesium oxide and aluminum oxide nanoparticles,[4-9] although activity has also been found for other oxides, such as calcium oxide.[10]
Detoxification of HD (Mustard Gas)
The toxicity of mustard gas (Fig. 1) is because of its capability to attach simultaneously and irreversibly to two biomolecules. The reaction takes place by an SN mechanism with an intermediate sulfonium cation as a key reaction species.
Magnesium oxide has a considerable number of basic sites that interact with the HD by hydrohalogen elimination, forming vinyl chloroethyl sulfide, or by exchange of chlorine with a hydroxyl group from the magnesium oxide surface.[6] The reaction proceeds further to divinylsulfide and in the other case to the corresponding glycol. The final ratio between the two major products was found to be approximately 50:50. The half-life of HD in this first-order reaction is 17.8 hr.[6] According to a careful kinetic examination, the reaction actually starts much faster. However, it slows down when part of the surface becomes clogged with residues of the reaction, which are permanently bound to the surface. Thus the limiting step in the reaction is the diffusion of the viscous and relatively low-volatility HD among magnesium oxide aggregates. This study has shown that the half-life of warfare agents is proportional to their vapor pressure—the higher the vapor pressure is, the higher the diffusion rate and hence the reactions leading to decontamination will be.[6] When calcium oxide was tested instead of magnesium oxide, the ratio of the products changed significantly to 80:20 elimination/hydrolysis products.[10] Elimination was the preferred route for the reaction, although it depended significantly on the way the sample was pretreated: When the nanoparticles were dried in advance before their contact with HD, elimination to hydrolysis products changed completely to 5:95 elimination/hydrolysis pro-ducts.[10] It is particularly important to note that a catalytic decomposition process was found for CaO nanoparticles.[10]
Additional experiments have shown[7] that dispersion of nanoparticulate magnesium oxides in a variety of solvents with/without addition of small amounts of water can alter the rate as well as the product types and their ratio. The most prominent effect observed was that the solvent speeds up the reactions because it eliminates the diffusion problem caused by the relatively high viscosity of warfare agents. Another important feature is that Lewis and Bronsted acid sites work simultaneously with Lewis base sites to decompose HD and HD stimulants.[7]
Another oxide that can be synthesized with high surface area using an aerogel procedure is aluminum oxide (AP-Al2O3). Although aluminum oxide is not as basic as magnesium and calcium oxides, it possesses a large number of Lewis acid sites, which accelerate hydrolysis processes.1-5-1 Because nucleophilic centers are much less abundant, elimination is less prominent and the ratio of the elimination/hydrolysis products is 17:83, with the major product being dithioglycol, as observed by solid-state nuclear magnetic resonance (NMR).[5]
Detoxification of nerve agents (VX, GD, GB, and paraoxon)
The interaction of nerve agents such as VX and GD (soman) (Fig. 1) with nanosized magnesium oxide particles (AP-MgO) was tracked by solid-state NMR.[6] It allowed the kinetics of interaction as well as the intermediate products to be determined. The width of the peaks was used to judge whether a particular product or intermediate was attached to the surface: Species attached to the surface have broader peaks because of anisotropic effects. In the case of GD, hydrolysis was the major process that took place, yielding GD acid (pinaco-lylmethylphosphonic acid) as a major product and methyl-phosphonic acid as a secondary product. According to NMR data, both products strongly interact with the surface, most probably by strong ionic interaction.1-6-1 The basic surface of the magnesium oxide nanocrystals most certainly converts the products to their respective anions, which bind with the positively charged surface. A quick start of the reaction, followed by a slowing down to a constant rate of t1/2=28 min, is observed, as in all cases of interactions of nanomaterials with viscous warfare agents.
The VX agent is a thioester with high viscosity and lower volatility compared with GD. It slowly hydrolyses to ethyl methylphosphonic acid and methylphosphonic acid. It is noteworthy to mention that the toxic hydrolysis product, EA-2192 (the VX toxin with the ethyl group replaced by hydrogen), which usually forms by hydrolysis in water, is not observed when contacted with AP-MgO nanoparticles. A very similar behavior was found in the interaction of VX and GD with nanosized calcium oxide.[10]
Nanoscale-sized aluminum oxide interacts with nerve agents with a very different rate: Although the reactive and relatively volatile GD has a t1/2= 1.8 hr after a quick start-up, the VX agent’s t1/2 is above 6 days. The difference from the MgO case is that the interaction with the warfare agents erodes the surface, allowing the ”bulk” of Al2O3 nanoparticles to participate in the detoxification process. This makes aluminum oxide a very promising material for warfare agent cleaning because this erosion behavior, coupled with its high surface area, allows a very high reaction capacity.
BIOLOGICAL WARFARE DECONTAMINATION USING NANOMATERIALS
Biological warfare agents could be several completely different types: bacteria, fungi, viruses, or toxins. All these have major differences in their behavior, and their decontamination procedures can vary significantly. This makes creating a single total decontaminating material practically impossible.
There are several methods recognized so far in decontaminating biological warfare agents. The most widely applied method is disinfecting solutions, such as diluted bleach or chloramine T solution. They have the advantage of quick and complete disinfection of bacteria, viruses, and certain fungi. However, it has very significant disadvantages, such as aging, which deteriorates disinfecting activity. Another disadvantage is that the bleach solution exhibits a strong smell, is corrosive, and is safely applicable only for the most durable surfaces. There are studies which reveal that in some respects, bleach effectiveness can be overrated,[11] especially for aged solutions.
A limited method for decontamination is applying a gas such as chlorine or chlorine dioxide. It cannot be applied in open spaces and on sensitive equipment, and can permanently damage many surface types.
Another approach is the use of oil-in-water micro-emulsions, developed by Hamouda at al.[12,13] and Hamouda and Baker.[14] They have demonstrated good activity against certain spores and bacteria, but they have the disadvantage of being difficult to remove after the decontamination procedure. In addition, they cannot be used for filtering airborne pathogens.
An alternative to the aforementioned approaches is the application of very fine solid powders in the form of nanoparticles. They are advantageous for a decontamination procedure because they can be easily collected from surfaces by simple vacuum cleaning, and they can be applied on sensitive electronic and other equipment because no liquid is present; they are much less corrosive than any of the aforementioned methods as well.
Nanoparticles such as AP-MgO, ZnO, or CaO have limited bactericidal activity against most biological warfare agents, although they have decent activity against vegetative bacteria such as Escherichia coli, Bacillus cereus, and B. subtilis.[15] An outstanding property of nanoparticles is the demonstration of their capability to remove powerful biological toxins such as aflatoxins.[15]
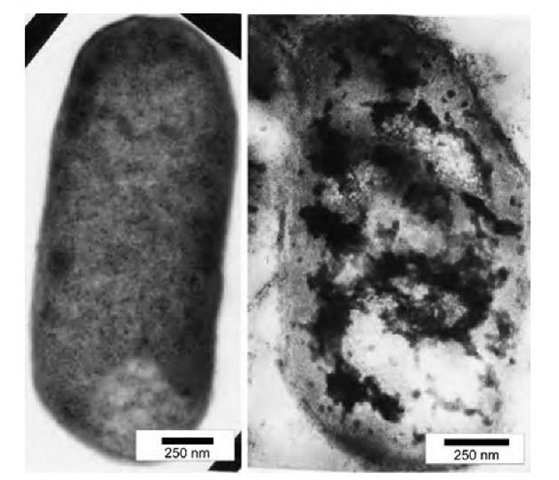
Fig. 2 Nontreated E. coli (left) and E. coli treated with AP-MgO/Cl2 nanoparticles (right). The dark matter in the right micrograph represents nanoparticles that penetrated inside the cell.
As was discussed in the ”Introduction,” nanoparticles feature huge surface areas and higher surface reactivity compared with bulk materials. Thus nanoparticles have very high adsorption activity per mass adsorbent, which allows adsorption of potent bactericides such as elemental chlorine, bromine, or iodine. Halogens are excellent bac-tericides in general. However, their use is restricted because of their high volatility, corrosive action, and toxicity.
Nanoparticles are capable of adsorbing as much as 6-10 wt.% free chlorine, 13-15 wt.% bromine, and 20-30 wt.% iodine. The adsorbates are preserved as halogens on the surface, preserving their properties as oxidizers, including their excellent bactericidal and sporicidal properties.1-16-1
The halogen-loaded magnesium oxide nanoparticles were found to preserve their activity even in water suspension,1-16-1 or in the dry state. If suspended, all types of halogen-loaded nanoparticles become positively charged, thus being attracted to the negatively charged (in general) bacteria or spores.[16] It was demonstrated by laser confocal microscopy that AP-MgO/X2 (X=Cl, Br, none) coagulates spontaneously with the bacteria in clumps composed of both bacteria and nanoparticle aggregates. When observed with transmission electron microscopy (TEM) and atomic force microscopy (AFM), microbial spores (B. subtilis) and vegetative cells (E. coli) were severely damaged (Fig. 2). In the case of more vulnerable vegetative cells, nanoparticles were found to enter the cells through holes developed in the membrane, thus bringing the adsorbed halogen inside the cell. The cell membrane and the spore coat were significantly damaged and eventually killed the cell by allowing its internal content out of the cell envelope.
An important feature of nanoparticles for their spori-cidal activity is their basicity: It was demonstrated that pretreatment of spores with basic solution removes partially or completely the thin outermost layer composed of base-sensitive proteins, which is a strong barrier for conventional sporicides, including free halogens.[17] Apparently, the nanoparticles of magnesium oxide have several properties, which render them as highly active bactericides and sporicides. They are abrasive (which is important for damaging the cell membrane), basic (important for partial spore coat removal), oppositely charged to the bacteria and spore cells, and carry a significant amount of oxidizing potential in the form of a potent bactericide (chlorine or bromine). For example, chlorine-loaded magnesium oxides carry as much as 10 wt.% active chlorine, whereas in comparison, undiluted bleach contains 6 wt.% active chlorine.
CONCLUSION
Nanoparticles constitute a new realm of the matter, which has properties very different from those of the bulk material. Because of higher surface-to-bulk ratios for the constituting atoms or ions and the much higher overall surface per unit weight, they exhibit outstanding chemical, physical, and biological properties. Their high surface area and activity may find use in ”hasty” decontamination of warfare agents. Nanoparticles of metal oxides can be pelletized and used as an additional protective layer for standard activated carbon filters. Furthermore, nanoparticles detoxify adsorbed warfare agents. Nanoparticulate magnesium oxide and its halogen-loaded derivatives, in particular, were found to possess bactericidal, viricidal, and sporicidal properties as well as capabilities to adsorb and deactivate complex toxins such as aflatoxins.
Nanoparticles have the potential to be used as an ”all-in-one” solution for both chemical and biological warfare decontamination.