Introduction
The life expectancy of patients with polycythemia vera (PV) and essential thrombocythemia (ET) is strongly affected by disease-related hemostatic complications, including thrombosis and, to a lesser extent, hemorrhages. Reported incidence of thrombosis ranges from 12% to 39% in PV and from 11% to 25% in ET. The clinical manifestation of thrombosis varies from microcirculatory disturbances to most serious complications, like arterial and venous thromboses (Table 6.1).
Arterial thrombosis, which accounts for 60-70% of the events, includes ischemic stroke, acute myocardial infarction, and peripheral arterial occlusion. Events involving the venous system are represented by deep venous thrombosis of the lower extremities, pulmonary embolism, and intra-abdominal (hepatic, portal, and mesenteric) and cerebral vein thrombosis. The prevalence of the latter is unusually high among patients with PV or ET and is often the presenting feature of these diseases before diagnosis. Typical, but not exclusive, of ET is the involvement of the micro-circulatory system and it manifests as eryth-romelalgia, transient ischemic attacks, visual or hearing transitory defects, recurrent headache, and peripheral paresthesia.
ET and PV management remains highly dependent on the patient’s thrombotic risk.Since the use of myelosuppressive drugs (hydroxyurea) can reduce the rate of thromboses and hemorrhages in these subjects but can also accelerate the rate of leuke-mic transformation, a risk-oriented management strategy is highly recommended in these patients. Older age (>60 years) and previous thrombosis are well-established cardiovascular risk factors for thrombosis in these patients. The absence of both of these two risk factors identifies the low-risk patients. There is great attention in moving beyond these recognized risk factors, particularly in the young or asymptomatic low- and intermediate-risk individuals not without risk of thrombosis. Recently, the impact of new risk factors, such as leukocytosis and JAK2V617F mutational status and/or mutational burden, is under active investigation.
Table 6.1 Hemostatic disturbances described in patients with ET and PV
|
Arterial thrombosis |
|
Myocardial infarction |
|
Unstable angina |
|
Ischaemic stroke |
|
Transient ischaemic attack |
|
Acute peripheral and visceral thromboembolism |
|
Venous thrombosis |
|
Deep venous thrombosis (legs and arms) |
|
Pulmonary embolism |
|
Fatal cerebral sinus and venous thrombosis |
|
Unusual sites venous thrombosis (visceral vein |
|
thrombosis and cerebral sinus and venous thrombosis) |
|
Superficial venous thrombosis |
|
Micro-circulation disturbancies |
|
Erythromelalgia |
|
Seizures |
|
Migraine |
|
Vertigo |
|
Tinnitus |
|
Scintillating scotomas |
|
Amaurosis fugax |
Even in the absence of thrombotic manifestation, ET and PV patients present with a hyperco-agulable state, which is a laboratory finding of increased levels of plasma biomarkers of hemo-static system activation (Falanga et al. 1994, 2000; Posan et al. 1998; Wieczorek et al. 1995). Thus, an acquired thrombophilic state develops in these patients, who are prone to vascular complications, but the mechanisms ultimately responsible for activation of blood coagulation and the increased thrombotic tendency in ET and PV have not yet been elucidated.
Pathogenesis of Thrombosis
The pathogenesis of thrombosis and of the activation of blood coagulation in ET and PV is complex. Among other factors, a prominent role is played by abnormalities of the erythrocytes, platelets, and leukocytes, arising from the clonal proliferation of hematopoietic progenitor cells. These abnormalities involve not only quantitative changes in the number of these cells, which cause hyperviscosity secondary to the increased blood cell mass, but also qualitative changes in the molecular characteristics of these cells. Prothrombotic factors expressed by transformed vascular cells (i.e., platelets, red blood cells, and leukocytes) include: (a) the production of procoagulant and proteolytic properties, (b) the secretion of inflammatory cytokines, and (c) the expression of adhesion molecules. In addition, the endothelial injury, caused by both hyperviscosity and by leukocyte-derived proteases (i.e., elastase, cathepsin G, and myeloperoxidase), may also predispose to thrombosis by upregulating endothelial adhesion molecules, which mediate platelet and leukocyte adhesion to vascular cells, and localize the secretion of thrombogenic and angiogenic pep-tides released by inflammatory cells. Recently, other two possible mechanisms of systemic hyper-coagulability have been identified: an increased production of procoagulant microparticles and the occurrence of an acquired activated protein C resistance.
Red Blood Cell Abnormalities
Erythrocytosis
The prothrombotic effect of an elevated hemat-ocrit has been clearly demonstrated in PV patients by the observation that at progressively higher hematocrit values, there is an increase of throm-botic risk (Pearson and Wetherley-Mein 1978). Hematocrit around the generally accepted upper limit of normal may be an important factor in the causation of occlusive vascular diseases, particularly in the cerebral circulation, as it determines an increase in blood viscosity (Adams et al. 2010) . It has been observed that at high hematocrit values (47-53%), the cerebral blood flow is significantly lower than at hematocrit values in a lower range (36-46%). In addition, the reduction of hematocrit by venesection increases the flow by a mean of 50% largely due to a reduction in viscosity (Thomas et al. 1977). In untreated PV subjects, most thrombotic accidents occur in the cerebral circulation, particularly sensitive to blood hyperviscosity (Kwaan and Wang 2003).
At high shear rates, the raise of the red cell mass displaces the platelets toward the vessel wall, thus facilitating shear-induced platelet activation and further enhancing the increased platelet-platelet interactions (Huang and Hellums 1993; Turitto and Weiss 1983). In addition, at low shear rates, as it occurs in venous flow, hyperviscosity can increase the thrombotic risk by causing a major disturbance of blood flow. A proper management of blood hyperviscosity is essential but does not abolish the in vivo platelet activation and the increased thrombotic risk existing in PV subjects (Landolfi et al. 1992).
Red Blood Cell Functional Disorders
In addition to increased red blood cell count, in ET and PV biochemical changes in the cell membrane and content (Turitto and Weiss 1980) of these cells have been reported. These changes may independently impair blood flow also through the formation of red blood cell aggregates which have the potential to directly block blood flow in small vessels, thus contributing to cause ischemia and infarct, especially in the cerebral blood flow, and to facilitate platelet-leukocyte interaction with the vessel wall (Pearson and Lipowsky 2000; Yedgar et al. 2002).
Platelet Abnormalities
Thrombocytosis
The role of thrombocytosis in the pathogenesis of thrombotic events is controversial. Although clinical improvement of microcirculatory disturbances and/or improved platelet function after control of thrombocytosis have been reported, no clear correlation of thrombocytosis with risk of major cardiovascular events has been demonstrated.For example, both in the PVSG and the ECLAP (Landolfi et al. 2004) prospective study, the platelet count did not predict for thrombosis occurrence. Finally, the results of the only randomized trial to date (the primary thrombocythemia 1 [PT1] study), which randomized high-risk ET patients to hydroxyurea (a global myelosuppressive agent) or anagrelide (a platelet-only-reducing agent), showed that, despite similar control of platelet count by either drug, indicated that the composite primary end point (arterial or venous thrombosis, serious hemorrhage, or death from vascular causes) occurred more often in recipients of anagrelide plus aspirin than in those receiving hydroxyurea plus aspirin (Harrison et al. 2005). The global effects of hydroxyurea on other blood cell populations, i.e., leukocytes, other than platelets, might underlie the correlation between control of platelet count and reduction of thrombosis rate observed in the first prospective study in high-risk patients with ET by Cortelazzo et al. (1995). Differently, in primary myelofibrosis (PMF), a condition characterized by less frequent cardiovascular events than in PV or ET, a correlation between thrombocytosis and thrombosis was found (Cervantes et al. 2006).
Extreme thrombocytosis (i.e., platelets >1,500 χ 109/L), on the other hand, can favor hemorrhagic rather than thrombotic manifestations in ET patients (Cortelazzo et al. 1995) .This paradox has been attributed to the possible occurrence of an acquired von Willebrand syndrome, due to an increased clearance of the large von Willebrand factor multimers from plasma (Landolfi et al. 2006). Although generally asymptomatic, the same phenomenon has been reported occasionally in secondary thrombocytosis, particularly after splenectomy. Platelet count reduction proved successful in normalizing the plasma von Willebrand multimer pattern and in reducing bleeding tendency.
Platelet Functional Abnormalities
While the data on the role of thrombocytosis in the pathogenesis of thrombosis in ET and PV are not conclusive, there are several other lines of evidence for a contribution of platelets to thrombotic risk. For example, in patients with ET, a role of platelets in mediating microvessel occlusions (i.e., eryth-romelalgia) is suggested by the prompt relief of symptoms with aspirin, the normalization of tests measuring in vivo platelet activation (Michiels et al. 2006) . In contrast to the inefficacy of coumadin, control of platelet function with low-dose aspirin and reduction of platelet counts to normal prevented the recurrence of microvascular circulation disturbances in the endarterial microvasculature of the cerebral, coronary, and peripheral circulation. Furthermore, low-dose aspirin significantly reduced the risk of cardiovascular events in PV as demonstrated by the ECLAP randomized clinical trial. Patients randomized to receive aspirin had a 60% reduction of combined end point of nonfatal acute myocardial infarction, nonfatal stroke, or death from cardiovascular causes (Landolfi et al. 2004).
The peculiar characteristics of thrombohem-orrhagic diathesis in ET and PV prompted the design of many in vitro studies to demonstrate and characterize possible platelet abnormalities. In the past, numerous platelet defects have been identified in ET and PV patients. The majority of these observations were related to a decreased functionality and included abnormal platelet aggregation, reduced levels of membrane adhesion molecules (i.e., glycoproteins Ib, IIb/IIIa, IV, and VI), acquired storage pool disease, and defective platelet metabolism (i.e., abnormal arachidonic acid metabolism) (Landolfi et al. 1995; Schafer 1984). To the opposite, more recent studies have shown that platelets from these patients circulate in an activated status, as assessed by the detection of increased expression on their surface of P-selectin and tissue factor (Arellano-Rodrigo et al. 2006; Falanga et al. 2005b). An enhanced in vivo platelet activation in ET and PV patients is further suggested by the finding of increased levels of platelet activation products both in plasma (i.e., beta-thromboglob-ulin and platelet factor 4) and urine (i.e., throm-boxane (Tx) A2 metabolites 11-dehydro-TxB2 and 2,3-dinor-TxB2) (Jensen et al. 2000; Landolfi et al. 1992).
Once activated, platelets provide a catalytic surface for the generation of thrombin, which further amplifies platelet activation. Recently, our group demonstrated in patients with ET and PV, and particularly in those carriers of the JAK2V617F mutation, an increased thrombin generation capacity of platelets, which was associated to the occurrence of platelet activation. The cytoreduc-tive therapy with hydroxyurea significantly affects this prothrombotic phenotype (Panova-Noeva et al. 2011).
White Blood Cell Abnormalities
Leukocytosis
In the recent years, many retrospective studies have identified leukocytosis as a potential risk factor for arterial and venous thromboses in patients with ET and PV (Carobbio et al. 2008, 2007; Landolfi et al. 2007; Palandri et al. 2011). In addition, leukocytosis was also identified as a risk factor for recurrent arterial thrombosis in young (i.e., <60 years) ET and PV patients (HR for arterial recurrence 3.35, 95% CI 1.22-9.19) (De Stefano et al. 2010) . Of note, in sickle cell patients, an increased baseline white cell count has been found to be an independent risk factor for acute chest syndrome and cerebral infarction, and quantitative and qualitative reductions in leukocytes during hydroxyurea treatment were correlated with a better disease outcome (Stuart and Nagel 2004) . Differently, a retrospective study showed that leukocytosis at diagnosis (defined by a cutoff level of either 15 or 9.4 χ 109/L) did not appear to influence the risk of thrombosis in low-risk ET or PV patients (Gangat et al. 2009). Prospective clinical studies with stratification of patients according to their baseline leukocyte counts are needed to definitely classified leuko-cytosis as a prognostic risk factor.
Leukocyte Qualitative Abnormalities
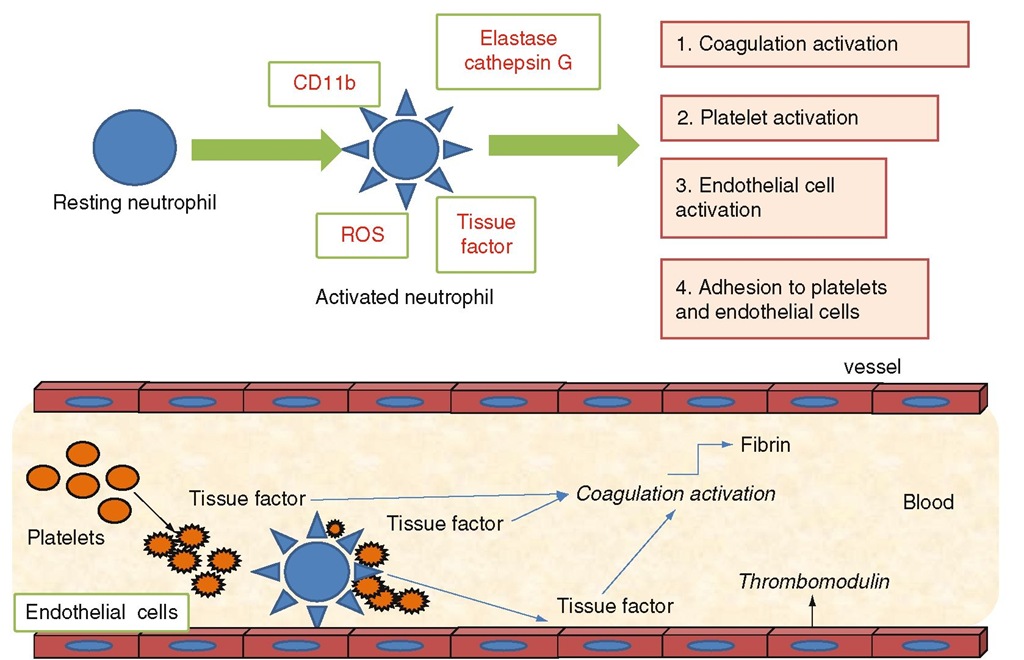
Leukocytes can contribute to the pathogenesis of thrombosis in ET and PV through recently discovered mechanisms of activation and interaction with platelets and endothelial cells and the coagulation system. In addition, leukocytes may contribute to inflammatory processes in atherosclerotic plaques and, in this way, increase the probability of vascular events (Falanga et al. 2005a; Marchetti and Falanga 2008). As neutrophils represent the most abundant proportion of the circulating leukocytes in ET and PV, a role for neutrophils in thrombosis has been hypothesized. Neutrophils have a central role in the inflammatory response and also in linking the inflammatory response to the activation of blood coagulation (Falanga et al. 2005a). Once activated, neutrophils produce reactive oxygen species (ROS), release proteolytic enzymes from their cytoplasmic azurophilic granules (elastase, cathepsin G), and express higher and functional levels of the ß2 integrin Mac-1 (or CD 11b) on their cell surface (Fig. 6.1I. All of these molecules can affect the hemostatic system and induce a prothrombotic condition (Falanga et al. 2005a; Afshar-Kharghan and Thiagarajan 2006). Notably, the fact that activated neutrophils can induce a hypercoagulable state in vivo has been well demonstrated by a study of a group of healthy donors administered granulocyte colony-stimulating factor (G-CSF) for the mobilization and collection of peripheral blood progenitor cells (Falanga et al. 1999). G-CSF caused the activation of neutrophils in these subjects, which was associated with a parallel increment in plasma levels of markers of activation of blood coagulation and endothelium. These effects were transient, as they persisted as long as the growth factor was administered (i.e., 5-6 days), and normalized after G-CSF withdrawal.
Fig. 6.1 Interaction of neutrophils with the hemostatic system. Molecules which specifically increase during the acute and chronic inflammatory disease (i.e., N-formyl-methionyl-leucyl-phenylalanine or fMLP, complement factors, cytokines, and growth factors) can activate the biological and metabolic functions of neutrophils, including adhesion and migration through the endothelium, phagocytosis, and oxidative killing. These activities represent the neutrophil response to inflammatory injury. On the other hand, activated neutrophils can also affect the hemostatic system through the same pathways. Particularly, the release of proteolytic enzymes (i.e., elastase, cathepsin G) and of reactive oxygen species (ROS) can activate/damage platelets and endothelial cells and impair some coagulation proteins. Elastase can prote-olytically inactivate several plasma physiological inhibitors of blood coagulation and, on the other side, cleave and activate some procoagulant factors (i.e., factor V and factor X). Both elastase and cathepsin G can cause the detachment or lysis of endothelial cells and also modify endothelial cell functions involved in thromboregulation. The adhesion of neutrophils to other blood cells allows the formation of a close microenvironment where the enzymes released by neutrophils are protected against plasma inhibitors and can consequently easily act on their substrates. In addition, cathepsin G is a very potent platelet agonist. Activated platelets express P-selectin and TF and release microparticles. The increased expression of CD11b on neutrophil surface allows the adhesion of neu-trophils to endothelial cells and platelets and the assembly of coagulation proteases on the neutrophil surface. Finally, neutrophils can express TF, even though the origin of neu-trophil TF is not yet clarified, and provide an alternative pathway for thrombin generation
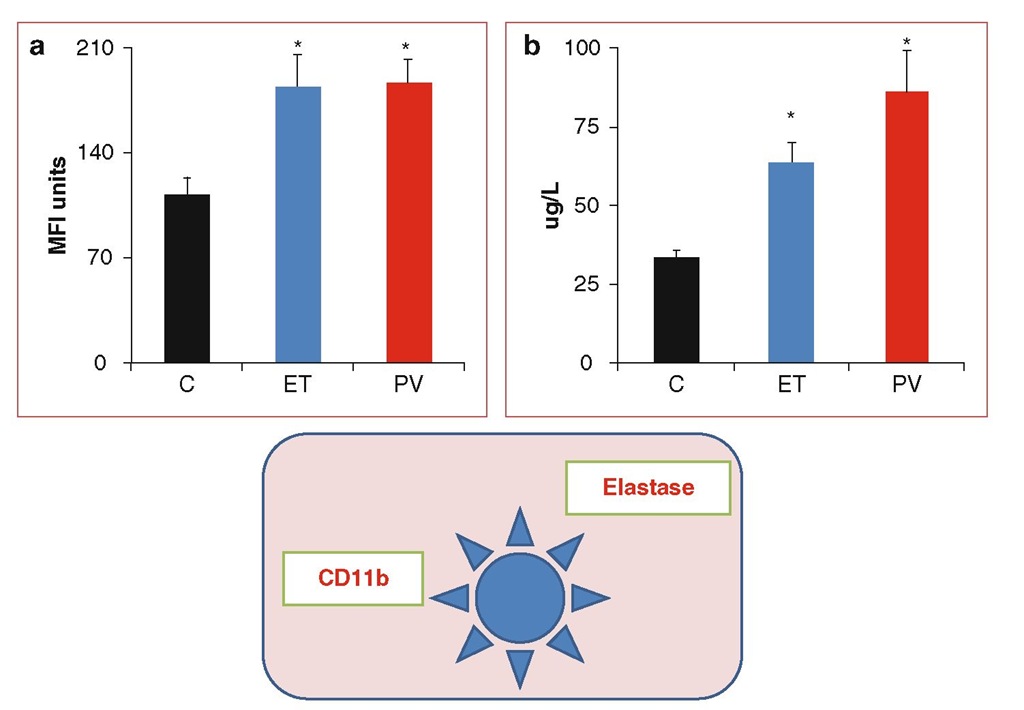
Fig. 6.2 Neutrophil activation. Cytofluorometric analysis of the expression of CD11b on the neutrophil cell surface (panel A) and measurement of neutrophil elastase concentration in plasma (panel B) showed a significant increment of this activation marker in patients with ET and PV compared to healthy control subjects (C). * p < 0.05 versus C.
In patients with ET and PV, the occurrence of neutrophil activation was demonstrated by the detection of specific phenotypical changes (increment in CD 11b) and by the measurement of increased plasma concentration of neutrophil granule proteases (i.e., elastase and myeloperoxidase) (Falanga et al. 2000, 2005b) (Fig. 6.2). The abnormalities of neutrophils directly correlated with the increase in plasma levels of biomarkers of blood coagulation and vascular endothelium activation, supporting a possible involvement of neutrophils in the pathogenesis of the hyperco-agulable state in these disorders. Several studies have described increased levels of circulating platelet/neutrophil aggregates in ET and PV patients and have attributed this phenomenon to both platelet (Jensen et al. 2001; Alvarez-Larran et al. 2004; Villmow et al. 2002) and neutrophil activation (Falanga et al. 2005b). Interestingly, in ET patients receiving aspirin, the increments in CD11b expression and in neutrophil/platelet aggregates induced after in vitro neutrophil activation were significantly lower versus nonaspi-rin-receiving ET subjects, suggesting that aspirin treatment may inhibit the interaction between neutrophils and platelets.