Introduction
Neurodegenerative conditions are increasing in prevalence as the average human life expectancy rises. Alzheimer’s disease (AD) is the fourth commonest cause of death in the United States; the recent outbreak of new variant Creutzfeldt-Jakob disease (nvCJD) has raised the specter of a large population being at risk to develop this prionosis. The pathogenesis of many neurodegenerative diseases is now recognized to be associated with abnormalities of protein conformation. A common theme in these disorders is the conversion of a soluble normal precursor protein into an insoluble, aggregated, P-sheet rich form that is toxic. In AD, a critical event is the conversion of the normal, soluble Ap (sAP) peptide into fibrillar Ap, within neuritic plaques and congophilic angiopathy (1). Similarly, in the prionoses, the central event is the conversion of the normal prion protein, PrPC, to PrPSc (2). An increased P-sheet content characterizes both Ap and PrPSc.
AD and many of the prionoses are forms of cerebral amyloidosis. Several other diseases also fall into this category, and include familial British dementia (3), familial Danish dementia, and familial Hungarian amyloidosis (4). In these cerebral amyloidoses, either a mutation occurs in a systemically expressed precursor protein, which increases the propensity of the entire protein and/or a degradation fragment to adopt a P-sheet conformation, or this conformational change can occur spontaneously.
Several other neurodegenerative diseases, which are not amyloid diseases, also have a similar pathogenesis (Table 1). For example, Huntington’s disease and the spinocerebellar ataxias are associated with increased CAG repeats in the Huntington and ataxin genes respectively (5,6).
Table 1
Conformational Neurodegenerative Conditions Characterized by Abnormal Protein Structure
|
Disease |
Protein |
Normal structure |
Abnormal structure |
abnormal protein accumulation |
|
Prionoses |
Prion |
a-Helical and random coil |
P-Pleated |
Variable extracellular amyloid |
|
Alzheimer’s disease |
Amyloid p |
a-Helical and random coil |
P-Pleated |
Extracellular amyloid |
|
Familial British dementia |
Bri |
Mainly a-helical and random coil |
P-Pleated |
Extracellular amyloid |
|
Familial Hungarian dementia |
Trans-thyretin |
Mixture of a-helical and p-pleated |
P-Pleated |
Extracellular amyloid |
|
Frontotemporal dementia |
tau |
Soluble |
Aggregated |
Cytoplasmic |
|
Huntington’s |
Huntingtin |
<35 CAG repeats |
>36 CAG repeats |
Nuclear |
|
Parkinson’s |
a-Synuclein |
Soluble |
Aggregated |
Cytoplasmic |
|
Spinocerebellar ataxia |
Ataxin |
Few CAG repeats |
Many CAG repeats |
Nuclear |
|
DRPLA |
Atrophin-1 |
<36 CAG repeats |
>49 CAG repeats |
Nuclear |
|
SBMA |
Androgen receptor |
<36 CAG repeats |
>40 CAG repeats |
Nuclear |
DRPLA, dentatorubral and pallidoluysian atrophy; SBMA, spinal and bulbar muscular atrophy.
These CAG repeats result in increased lengths of glutamine residues, which enhance the propensity for the mutant protein to aggregate and form intracellular neuronal inclusions that produce toxicity. The recognition of the importance of abnormal conformation in these disorders has led to the development of both anti-^-sheet compounds and immunological approaches that affect the conversion into the pathological conformer and/or the clearance of the disease-associated proteins. These approaches hold promise as potential therapeutic strategies in this category of illness.
p-Sheet Breaker Peptides and the Prion Protein
A number of compounds have been tried in the treatment of prion diseases, including Congo red (7,8), anthracyclines (9), amphotericin B (10,11), and sul-phated polyanions (12). Some of these have been shown to delay the incubation times of animals infected with scrapie, but these agents have limitations in terms of toxic effects and/or unfavorable pharmacokinetic properties. We have recently designed a number of compounds that interact with the PrPSc structure and act as p-sheet breakers (13). These are short, synthetic peptides, which, because of sequence homology, bind to PrP. Prolines (Pros) were introduced into these short peptides, since prior data suggests that the presence of these residues inhibits a P-sheet conformation (14-16). Pros are incompatible with a P-sheet conformation for a number of reasons, including the peptidyl-prolyl bond induced by the Pro ring does not fit with the peptide bond geometry found within p-sheet motifs, and the Pro ring sterically hinders the P-sheet bonding network. The PrP sequence picked for designing the P-sheet breaker corresponded to residues 115-122, because this region has been implicated in the conversion process of PrPC to PrPSc (17-19).
A number of different PrP homologous peptides (some of which are shown in Table 2) were first screened for inhibitory activity on the conversion of PrPC to PrPSc using an in vitro system. Synthetic peptides, corresponding to PrP residues 109-141, can reproduce some of the properties of PrPSc in vitro (17,18,20). The authors determined the ability of these various candidate P-sheet breaker peptides to inhibit amyloid-like fibril formation of PrP109-141, using a fluorometric assay based on the fluorescence emission of thioflavine T (21,22). Using this assay, a 13-residue peptide (iPrP13) had the greatest P-sheet-breaking capability (Fig. 1).
Using this peptide we were able to show that the proteinase K sensitivity of extracted mouse PrPSc, human PrPSc extracted from sporadic CJD patients, or from a nvCJD patient, was increased, in a concentration-dependent fashion, by iPrP13 (13). The in vivo effect of iPrP13 was also tested using the mouse-adapted scrapie strain 139A. Incubation time assays were done using three different 10-fold dilutions of extracted 139A PrPSc, in the presence or absence of an equimolar concentration of iPrP13. At each dilution, one group of mice was injected with untreated and nonincubated PrPSc, a second group was inoculated with PrPSc that was incubated for 48 h alone, a third group was inoculated with PrPSc and iPrP13 without incubation, and a fourth group was inoculated with PrPSc and iPrP13 following 48 h incubation. The iPrP13 induced a substantial delay in the appearance of disease (Table 3).
The above results suggest that p-sheet breakers may have therapeutic potential in the prionoses. Such an approach (Fig. 2) may have applicability to any
Table 2
The Sequence of p-Sheet-Breaker Peptides and Control Peptides Used
|
Peptide |
Sequence |
|
iPrP13 |
DAPAAPAGPAVPV |
|
iPrP11 |
DAAAPAGAPVV |
|
iPrP10 |
DAPAAPAVPV |
|
iPrP9 |
DAAPAAPVV |
|
iPrP8 |
DAPAAPVV |
|
iPrP7 |
DAAAPVV |
|
iPrP5 |
AAPVV |
|
CP1 |
GYITVAAVFRG |
|
CP2 |
PAADVPPAAV |
|
PrP101-119 |
KPSKPKTNMKHMAGAAAAG |
|
PrP109-122 |
MKHMAGAAAAGAVV |
Fig. 1. A number of different p-sheet-breaker candidate peptides (see Table 2) were tested for inhibition of amyloid-like fibril formation by synthetic PrP109-141. 30 ^g aliquots of PrP109-141 were incubated alone or with a 10 fold molar excess of the various peptides in 30 ^L 0.1 M Tris-HCl, pH 7.4, for 7 d at 37°C. Fibrillogenesis was quantitated by thioflavine-T fluorometic assay.
Table 3
In Vivo Effect of iPrP13 on the Incubation Times of Mouse-Adapted Scrapie Strain 139A
|
|
Incubation time (days) |
|||
 |
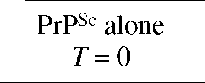 |
 |
 |
 |
|
100 |
129 ± 0 |
136 ± 5 |
143 ± 3 * |
148 ± 2* |
|
1000 |
145 ± 6 |
141 ± 7 |
159 ± 2 ** |
176 ± 9* |
|
10000 |
173 ± 12 |
162 ± 9 |
185 ± 13 |
225 ± 26** |
Incubation time assays were done using three different 10-fold dilutions of extracted 139A PrPSc, in the presence or absence of an equimolar concentration of iPrP13. At each dilution, one group of mice was injected with untreated and nonincubated PrPSc, a second group was inoculated with PrPSc that was incubated for 48 h alone, a third group was inoculated with PrPSc and iPrP13 without incubation, and a fourth group was inoculated with PrPSc and iPrP13 following 48 h incubation. The iPrP13 induced a substantial delay in the appearance of disease (*indicates P < 0.05 and ** indicates P < 0.06 vs PrPSc at the same dilution and incubation time).
Incubation time assays were done using three different 10-fold dilutions of extracted 139A PrPSc, in the presence or absence of an equimolar concentration of iPrP13. At each dilution, one group of mice was injected with untreated and nonincubated PrPSc, a second group was inoculated with PrPSc that was incubated for 48 h alone, a third group was inoculated with PrPSc and iPrP13 without incubation, and a fourth group was inoculated with PrPSc and iPrP13 following 48 h incubation. The iPrP13 induced a substantial delay in the appearance of disease (*indicates P < 0.05 and ** indicates P < 0.06 vs PrPSc at the same dilution and incubation time).
Fig. 2. Hypothetical scheme for anticonformational therapeutic agents. p-sheet breakers can function both to inhibit the formation of a p-sheet conformation, and to return the abnormal conformation to its physiological form (24,13). Immunization with the peptides homologous to the disease-associated protein can act to increase its clearance and/or degradation. Alternatively, antibodies to the disease-associated protein may also act to inhibit the conformation change to increased p-sheet, as has been shown in vitro for some anti-Ap antibodies, which disaggregate Ap peptides (37).
However, significant disadvantages of iPrP13 are that, as a short peptide, it is likely to be subject to extensive proteolytic degradation, and that it has poor permeability of the blood-brain barrier. These difficulties could be overcome by designing pepti-domimetic or pseudopeptides, based on the structure of iPrP13. This P-sheet- breaker concept also does not need to be limited to agents that are homologous to the physiological precursor protein. In that light, a recent report, using por-phyrin and phthalocyanine compounds, showed an increase in the survival times following inoculation of PrPScin experimental animals (23). The activity of these compounds was, like iPrP13, correlated with in vitro inhibition of PrPSc formation.
p-Sheet-Breaker Peptides and Amyloid p
Major features of AD are the deposition of Ap in the form of neuritic plaques and congophilic angiopathy, in which it is fibrillar and has a high p-sheet content. The Ap peptide also exists as a normal peptide in biological fluids, where it is called "soluble AP" (sAP), and is thought to have a more random coil and/ or a-helical secondary structure. Hence, a critical event in the pathogenesis of AD is the conformational change of sAp to Ap (1). This conformational change is analogous to the PrPC-to-PrPSc transition. Similar in concept to the authors’ design of iPrP13, the authors have also designed Ap homologous peptides, with Pro residues that inhibit a P-sheet conformation. Using an in vitro assay of Ap fibrillogensis, a five-residue peptide, iAp5, was found to have the greatest activity (15). In a rat brain model of Ap amyloidosis, iAp5 inhibited fibril formation when administered at the same time as an intracerebral inoculation of AP1-42. In rats in which the AP1-42 was injected without iAp5, large Congo red-positive, fibrillar deposits formed (16). These results indicated that peptides such as iAp5 could be used to prevent Ap deposits from forming. More recently, we have also used the rat model of AD to show that iAp5 can reverse existing deposits of AP1-42 (Fig. 3; 24). In this experiment, AP1-42 was first stereotactically injected into the amygdala of the rat and allowed to form fibrillar deposits over a period of 8 d. At that point, the rats were injected with iAp5, control peptides, or vehicle, followed by histological examination 8 d later. The rats injected with the iAp5 showed evidence of dissembly of the Ap fibrils that had formed in vivo (24). A number of other compounds have also been shown to dissemble Ap fibrils, using in vitro assays, such as melatonin (25,26), nicotine (27), apolipoprotein J (28), anthracycline (29), rifampicin (30,31), hexadecyl-N-methylpiperidinium(32), and Congo red (33). It remains to be determined if these will be active in vivo. With the current availability of various transgenic mouse models of AD that develop cerebral amyloid deposits, many of these compounds are now being tested for both prevention of amyloid formation, and for the disassembly of existing lesions.
Immune Response in the Prionoses and Alzheimer’s Disease
It has recently been shown that immunization of transgenic mice with AD-related neuropathology, using fibrillar AP1-42 as an antigen, reduces or prevents cerebral Ap amyloid deposits (34).
Fig. 3. Specific disassembly of Ap fibrils in vivo by iAp5, and subsequent reduction of cerebral Ap deposition. Ap-stained coronal sections (x100, original magnification) through the amygdala at the injection site, in rats treated with (A) Ap1-42/vehicle (VEH) and (B) Ap1-42/iAp5. Intra-amygdaloid injection of VEH or iAp5 was performed 8 d following injection of Ap1-42 into the same brain region, and the rats were killed 8 d later. (C) Quantitative analysis of Ap deposits at 16 d postoperatively in rats injected first with Ap1-42 (5.0 nmol), followed 8 d later with an injection of iAp5 (LPFFD), control peptides (CP5 [ETRGD], CP10 [ISEVKMDAEF]) (200.0 nmol), or VEH. Deposit area was measured by image analysis of Ap immunoreactive sections. Each bar represents the mean + SEM of N = 8-9 rats. Effect of iAp5 relative to the other treatment groups: ** P < 0.01.
The transgenic mice used in this experiment overexpress mutant human APP, and progressively develop Ap cerebral amyloid deposits in an age- and brain-region-specific manner (35).
When animals were inoculated with fibrillar AP1-42 at 6 wk, prior to the development of AD-related pathology, cerebral amyloid deposition was essentially prevented, but animals inoculated at 11 mo, with substantial amyloid deposits, had the AD-related pathology greatly reduced. These inoculations resulted in the generation of high anti-Ap antibody titers. The protective effect on cerebral amyloid deposition could be related to an inhibition of the conformational change from sAp to Ap (similar to the P-sheet-breaker peptides discussed above), or other effects, such as increased clearance of Ap and/or blocking of Ap/immune cell interactions, which are involved in amyloid formation. Prior in vitro data suggests that at least some anti-Ap antibodies can inhibit fibrillar aggregation of these peptides (36,37).
These experiments suggest that modulation of the immune system against aggregated, disease-associated proteins may represent a novel therapeutic approach. Furthermore, in another model system, it has recently been shown that an oral vaccine is effective at prevention of experimental stroke and epilepsy (38), illustrating the feasibility of a vaccination strategy that targets brain proteins. The importance of the immune system in the pathogenesis of AD has been further underscored by the recent reports of a genetic linkage between common population polymorphisms of the interleukin-1a and interleukin-1p genes (39,40). Interleukin-1 is an acute-phase proinflammatory cytokine, which has been implicated in neuroinflammatory pathways resulting from Ap cerebral deposition. Many laboratories, including that of the authors, are currently testing a number of different Ap epitopes in various transgenic AD mouse models, to assess this as a therapeutic approach. Our preliminary results using a variety of Ap peptide epitopes for immunization confirm that such a ‘vaccination’ approach results in a dramatic reduction in amyloid burden in transgenic AD mice. However, it remains unclear whether these interventions will be beneficial or detrimental to natural disease progression. Extensive evidence has implicated inflammatory pathways in AD (41); however, this has been previously proposed to be associated with neuronal damage and death related to complement activation, free-radical damage and microglial activation (41-44). Indeed, a number of studies have shown that patients who have a long history of taking anti-inflammatory agents have a lower incidence of AD (45-47). Hence, whether experimentally induced inflammation will be beneficial or harmful for AD is likely to depend on the type and extent of response generated by the various immunization protocols used.
Modulation of the immune system may also have the potential to be beneficial for the prionoses. The immune system has long been recognized to have an unusual role in prion infections. There are no specific humoral or immune responses against prions following infection (48,49); in fact, the immune system appears to help, rather than impair, the propagation of prions following peripheral exposure. Although PrPC is expressed at various levels in most tissues of the body, infectivity multiplies only within the nervous system and in the peripheral lymphoid organs (50). Mice with severe combined immunodeficiency (SCID) illustrate the paradoxical role of the immune system in prion infections. These mice are partially resistant to scrapie, after intraperitoneal or subcutaneous inoculation, in contrast to immunocompetent mice of the same strain, and to immunologically reconstituted SCID mice (51,52).
The nature and actions of the immune cell type(s) that support prion infection still remains unclear. The essential controversy concerns the respective roles of lymphocytes and follicular dendritic cells (FDCs) in the replication of prions and their transport to the nervous system. After fractionation of spleen cells by density gradient centrifugation, the highest infectivity, between the second and ninth weeks following mice inoculation with scrapie agent, was found in lymphocytes (53). However, it may be that this cell fraction also contained FDC, because of their close interactions with B-lymphocytes.
Ionizing radiations did not influence mouse susceptibility to scrapie, suggesting that quiescent cells may principally support prion replication (54). Because the scrapie susceptibility of SCID mice, after reconstitution with hematopoietic precursors, appears to be dependent on the restoration of a normal lymphoid architecture (51), and PrPSc is detected in FDC of CJD infected mice (55), FDCs are likely to be involved in the replication and accumulation of the scrapie agent. However, the occurrence of scrapie agent within FDC could reflect their potent antigen-capturing function (55). Tumor necrosis factor receptor-1-knockout mice, which lack FDC and germinal center reaction, have a normal susceptibility to scrapie after peripheral inoculation; mature B-lymphocytes, rather than other hematopoietic cell lineages, are required for the neuroinvasion (56). However, such results were not confirmed with another model of FDC deficiency: the tumor necrosis factor a-knockout mouse (57). A key role of FDC in PrPSc replication is strongly supported by recent results showing that the expression of PrPC by FDC, but not by hematopoietic lineages, is required for scrapie susceptibility after peripheral inoculation (57).
Regardless of the question of which cells of the immune system are involved in prion propagation, it is clear that immunomodulators can influence susceptibility to scrapie. Mitogenic stimulation by phytohemagglutinin or bacterial lipopolysaccharide made mice susceptible to doses of prions that are otherwise ineffective, and reduced the incubation time after peripheral inoculation by nearly 20% (58). On the other hand, administration of high doses of prednisone, immediately before and after intraperitoneal inoculation of mice with a scrapie-infected brain homogenate, resulted in prolonged incubation periods (59). Given the encouraging results in AD model transgenic mice (34), as discussed above, the question is raised whether a similar approach may also work in prion infection, using PrPSc peptide analogs as immunogens. Such experiments are currently ongoing.
Conformation as a Therapeutic Target
There is growing evidence that numerous neurodegenerative conditions have the same underlying pathogenetic mechanism, namely, a change in protein conformation in which the P-sheet content is increased. In AD, amyloid deposition, in the form of neuritic plaques and congophilic angiopathy, is driven by the conversion of sAp to AP; in the prionoses, the critical event is the conversion of PrPC to PrPSc. In AD and in the prionoses, as well as in other cerebral amyloidoses (such as familial British dementia [3]), the abnormal P-sheet protein accumulates in the extracellular space. We suggest that this common con-formational theme in these disorders, and the extracellular localization of the accumulating abnormal protein, make them highly amenable to therapeutic approaches based on experimental manipulation of protein conformation and clearance. We have outlined the design of a number of p-sheet breakers for both Ap- and PrPSc-related deposits. These initially designed peptides can serve as a starting point for further peptidomimetic or pseudopeptide derivatives, which can have optimized biological and pharmacokinetic properties. In addition, immune system activation, directed against the abnormal P-sheet peptide, can serve as a p-sheet breaker and/or to increase the clearance of the disease-associated protein. These conformationally based approaches appear to have the best promise for rationale therapies for this devastating group of disorders.
Summary
Abnormal protein conformation is increasingly being recognized as part of the pathogenesis of numerous neurodegenerative conditions. The common theme in all these diseases is the conversion of a normal cellular and/or circulating protein into an insoluble, aggregated, p-sheet-rich form that is deposited in the brain. The aggregated proteins can accumulate extracellularly, often in the form of amyloid, or intracellularly, producing inclusion bodies. These deposits are toxic, and produce neuronal dysfunction and death. A unique category of the conformational conditions are prion-related diseases (or prionoses), in which the etiology is thought to be related to conversion of the normal prion protein, PrPC, into an infectious and pathogenic form, PrPSc. However, the most common of these disorders is AD, in which the central event is thought to be the conversion of normal soluble amyloid p (sAP) into fibrillar Ap, in the form of neuritic plaques and congophilic angiopathy. Growing understanding of the mechanisms involved in this category of disease raises the possibility of therapeutic approaches based directly on the prevention and reversal of pathologic protein conformations. Possible approaches include synthetic P-sheet-breaker peptides, which the authors’ preliminary data suggest may be useful for both AD and the prionoses, as well as for immunological approaches in which an antibody and/or cell-mediated response is triggered against the aggregating abnormal protein.


![Specific disassembly of Ap fibrils in vivo by iAp5, and subsequent reduction of cerebral Ap deposition. Ap-stained coronal sections (x100, original magnification) through the amygdala at the injection site, in rats treated with (A) Ap1-42/vehicle (VEH) and (B) Ap1-42/iAp5. Intra-amygdaloid injection of VEH or iAp5 was performed 8 d following injection of Ap1-42 into the same brain region, and the rats were killed 8 d later. (C) Quantitative analysis of Ap deposits at 16 d postoperatively in rats injected first with Ap1-42 (5.0 nmol), followed 8 d later with an injection of iAp5 (LPFFD), control peptides (CP5 [ETRGD], CP10 [ISEVKMDAEF]) (200.0 nmol), or VEH. Deposit area was measured by image analysis of Ap immunoreactive sections. Each bar represents the mean + SEM of N = 8-9 rats. Effect of iAp5 relative to the other treatment groups: ** P < 0.01. Specific disassembly of Ap fibrils in vivo by iAp5, and subsequent reduction of cerebral Ap deposition. Ap-stained coronal sections (x100, original magnification) through the amygdala at the injection site, in rats treated with (A) Ap1-42/vehicle (VEH) and (B) Ap1-42/iAp5. Intra-amygdaloid injection of VEH or iAp5 was performed 8 d following injection of Ap1-42 into the same brain region, and the rats were killed 8 d later. (C) Quantitative analysis of Ap deposits at 16 d postoperatively in rats injected first with Ap1-42 (5.0 nmol), followed 8 d later with an injection of iAp5 (LPFFD), control peptides (CP5 [ETRGD], CP10 [ISEVKMDAEF]) (200.0 nmol), or VEH. Deposit area was measured by image analysis of Ap immunoreactive sections. Each bar represents the mean + SEM of N = 8-9 rats. Effect of iAp5 relative to the other treatment groups: ** P < 0.01.](http://what-when-how.com/wp-content/uploads/2011/08/tmpD56_thumb.jpg)
