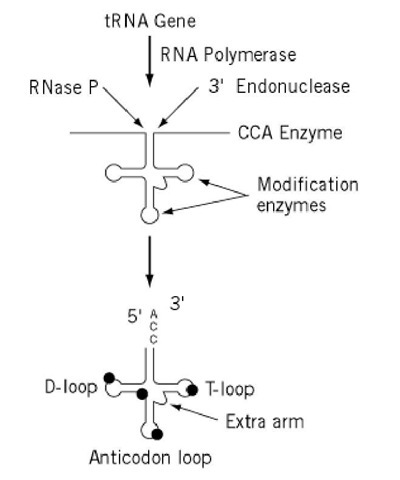
Depending on the organism and the organelle, any particular transfer RNA (tRNA) may be transcribed from the genome into an RNA molecule along with other tRNA, with messenger RNA (mRNA), or with ribosomal RNA (rRNA). Therefore, the first step in tRNA biosynthesis in many cases is the initial release of a precursor tRNA from such a polycistronic transcript. tRNA precursors share the need for certain posttranscriptional processing, either as part of the releasing process or subsequent to it. These shared needs include removal of the 5′-leader and 3′-trailing sequences and modification of bases (Fig. 1). Addition of the trinucleotide CCA at the 3′ end is thought of as a universal requirement, and it is in eukaryotes, because the CCA end is not encoded in the gene. In contrast, many prokaryotic tRNA gene transcripts do not require CCA addition as an initial processing event, because it is provided in the primary transcript and becomes the 3′-terminal sequence after other 3′-processing nucleases remove downstream nucleotides. Some tRNA require additional posttranscriptional alterations for their maturation. These include 5′-base addition, removal of intervening sequences, and RNA editing. While there are critical sequence-specific requirements for some RNA processing enzymes, it is safe to state the general rule that the most critical substrate characteristic for tRNA processing enzymes is retention of the overall tRNA structure in the precursor. It has also been shown that tRNA processing can proceed via alternative routes such that end processing can either precede or follow splicing (1) and splicing can begin with cleavage at either the 5′ or 3′ end of the intron (2) (see discussion below).
Figure 1. Schematic diagram of tRNA biosynthesis. Transcription produces a pre-tRNA that is trimmed on the 5′ end by RNase P and on the 3′ end by an endonuclease (or exonuclease) in preparation for CCA addition. Base modification reactions occur throughout the processing pathway. Some tRNA genes have intervening sequences which are not shown here but are described in the text.
1. Removal of 5′ Leader
The 5′ leader is removed from tRNA precursors by ribonuclease P (RNaseP), an enzyme found in all organisms and organelles in which tRNA genes are found. The cleavage reaction is a hydrolysis, requires both monovalent and divalent cations, and leaves a 5′-phosphate on the mature tRNA and a 3′-hydroxyl on the 5′ leader. Prokaryotic, eukaryotic and organelle RNaseP enzymes, with some possible exceptions, are ribonucleoproteins and require both the RNA and protein subunits for activity in vivo. Remarkably, the RNA subunit is responsible for catalysis in the bacterial enzymes (3) and is likely to serve the same function in eukaryotes. All RNaseP RNAs share short regions of sequence similarity and are predicted to take on similar elements of secondary structure. Although they can vary in size, most cluster in the 300-400-nucleotide range. The prokaryotic enzymes are about 10% protein, with a protein subunit of about 14 kDa (kilodaltons). Archaeal and eukaryotic enzymes are 50-70% protein, and the proteins identified so far are larger and share no sequence similarity to each other or to the prokaryotic proteins.
2. Removal of 3′ Leader
In some organisms, exoribonucleases perform 3′-end tRNA processing, while in others, endonucleases carry out that function. The best-studied exoribonucleases are from Escherichia coli. Six exoribonucleases that can trim tRNA precursors in vitro have been identified (4). Five can participate in 3′-end formation in vivo, but extensive functional overlap is demonstrated by the observation that cells retaining only one of these nucleases are viable. Complete 3′ processing in vitro required ribonuclease II and polynucleotide phosphorylase to shorten long 3′ trailers to intermediates extended by only two to four nucleotides. Final trimming by ribonulceases T and/or PH completes the process (5). The combined genetic and biochemical approaches of Deutscher and his colleagues have revealed that the relative importance of each exoribonuclease is different for different tRNA precursors, and that the combined action of the nucleases is most effective in maturing all tRNA. Whether exonuclease(s) or endonuclease(s) mature the 3′ end of nucleus coded tRNA in S. cerevisiae is still not established. However, examination of tRNA precursors and 3′ trailers from Drosophila (6), Xenopus (7), and silkworms (8) demonstrate that endonuclease cleavage prepares the 3′ end of nucleus-coded tRNA in these eukaryotes (9). Organelle tRNA precursor structure also establishes that 3′-end formation of mitochondrial and chloroplast tRNA is carried out by endoribonucleases. Indeed, these enzymatic activities have been measured in crude extracts and in some cases partially purified (10).
3. Addition of CCA 3′ End
ATP(CTP)-tRNA-specific nucleotidyltransferase adds the CCA end to tRNA, following 3′-end processing to provide the proper substrate. The enzyme also serves as a repair enzyme to restore the CCA end to tRNA victims of cellular nucleases and is required for this function regardless of whether the CCA end is gene-encoded. Since eukaryotic precursor tRNAs that contain intervening sequences have the CCA end, it is clear that the enzyme can bind partially processed tRNA. This is consistent with a model in which the enzyme interacts with tRNA at the corner of the tRNA where the T and D loops are juxtaposed and extends that interaction across the aminoacyl stem to the 3′ end (11).
4. Base Modification
Transfer RNA contains the highest concentration of modified bases of any type of RNA. There are 80 different nucleoside modifications known, and many display species and/or phylogenetic specificity (12). Modified bases are introduced after transcription and, depending on the enzyme, either precursor or fully processed tRNA can serve as the preferred substrates. In both prokaryotes and eukaryotes, characterization of precursors demonstrates that modifications do occur in a stepwise fashion (13, 14). Further enforcing an order of addition for nucleus-coded tRNAs, certain tRNA modification enzymes are confined to either the nucleus or the cytosol. Mutants deficient in a particular modification enzyme activity, so that a particular modification is not made, do complete subsequent modifications (10). Thus it is clear that an obligate order of modification per se is not required. Certain anticodon modifications are not found in unspliced precursors, suggesting that there is a requirement for splicing prior to the synthesis of the modified bases located in the anticodon region (15).
Histidyl tRNAs contain an extra 5′-G nucleotide relative to all other tRNAs. In E. coli, the G is derived transcriptionally, and RNaseP does not remove the extra G when it processes the 5′ end (16). In yeast, however, the 5′-most G in the histidyl tRNA is added by a histidyl tRNA guanylyltransferase in an ATP-dependent reaction (17). The 5′-G of spinach chloroplast histidyl tRNA is derived by transcription (18), whereas that of animal mitochondria is added posttranscriptionally (19).
5. Intron Removal
Since intervening sequences (introns) were discovered in yeast tRNA genes (20), the mechanisms involved in their removal have been sought. Splicing is initiated by an endonuclease that cleaves the pre-tRNA at the splice sites. Biochemical purification of the enzyme has been completed, and it is known to consist of three polypeptide subunits of 31, 42, and 51 kDa (21). Release of the intron leaves a 2′,3′-cyclic phosphate on the 3 end of the 5′ half of the tRNA (22), and a 5′-OH on the 5′ end of the 3′-half of the tRNA (23). The two half-molecules serve as the substrate for a tRNA ligase, which carries out the following steps: (i) creation of a 2′-phosphate by phosphodiesterase activity at the 2′,3′-cyclic phosphate; (ii) phosphorylation of the 5′-OH on the 3′ half by a polynucleotide kinase that uses GTP as the phosphate donor (24); (iii) transfer of AMP from the adenylylated form of the enzyme to the 5′-P on the 3′ half of the tRNA; and (iv) joining of the tRNA halves with the release of AMP (25). At this stage, the product contains a 3′,5′-phosphodiester linkage, as well as a 2′-phosphomonoester. The latter is removed by an NAD-dependent 2′-phosphotransferase in a reaction in which the 2′-phosphate displaces the nicotinamide part of NAD, to yield ADP-ribose 1”-2” cyclic phosphate (26). The RLG1 gene coding for the ligase has been characterized, and deletion analysis supports the idea that various activities can be attributed to separate domains in the protein (27). Similar enzyme activities are present in HeLa cell extracts, demonstrating that this is a conserved mechanism (28).
6. Editing
Like other RNA, tRNAs are substrates for editing, the remarkable process that alters the base sequence of an RNA after synthesis. So far, this type of tRNA processing has been identified only in mitochondria. Insertional editing in Physarum restores functional structure to mitochondrial tRNA (29). Substitutional editing also usually restores base pairing in stems. In Acanthamoeba castellanni A can be changed to G, U to G, and U to A, to correct mismatched base pairs (30). In the land snail, mitochondrial tRNAs sustain changes of C, U, and G to A that in all cases, except one, restore base pairing in the stem (31). This restoration of base pairing has been shown to be required for efficient removal of 5′ leaders and 3′ trailers in plant mitochondria (32, 33) consistent with the observation that tRNA-processing enzymes require at tRNA structure for optimal function. Finally, a C-to-U change in the second position of the anticodon of marsupial mitochondrial tRNAAsp is necessary for proper codon recognition (34). Although the mechanisms are not clear, there is no reason to expect that tRNA transcript editing is different from those editing processes operating on other RNAs.