In the absence of an energy source, a polymer will exist in equilibrium with a pool of monomers. In such an equilibrium, monomers will randomly associate and dissociate from the polymer, with no net change in length. Since many protein polymers (such as actin, tubulin, and RecA ) bind and hydrolyze a nucleotide cofactor, the possibility exists that this energy source can be used by the polymer to drive a flux of subunits through the filament. This will result in a steady state where the polymer is maintaining a constant length but moving subunits vectorially, a process known as treadmilling.
With no energy source, a polymer may have a "fast" growing end and a "slow" growing end, but the equilibrium constant (the ratio of the rate constants for subunit addition and loss) must be the same at both ends of the filament. Thus, if the rate of addition is tenfold greater at one end of the filament than at the other, the rate of subunit loss at this fast-growing end must be tenfold greater than at the slow-growing end. The critical concentration of the monomer (the concentration at which the monomer will be in equilibrium with the polymer, with no net growth or depolymerization) must be the same at both ends of the filament at equilibrium. In the presence of an energy source, it was first realized for actin (1) that ATP hydrolysis could lead to a situation where the critical concentrations are different at the two ends. There could thus exist a state where the monomer concentration leads to depolymerization at one end, but polymerization at the opposite end. Under these steady-state conditions, the filament length could remain constant, but there would be a net flux of subunits travelling through the polymer (Fig. 1).
A direct in vitro demonstration of the existence of treadmilling was shown for microtubules (2), but the role of treadmilling in vivo for both actin and microtubules has remained controversial for many years. Recent observations, however, have shown the existence of in vivo treadmilling for both actin (3) and microtubules (4, 5). The resulting vectorial movement of subunits through a polymer could be an important mechanism for motility and intracellular transport.
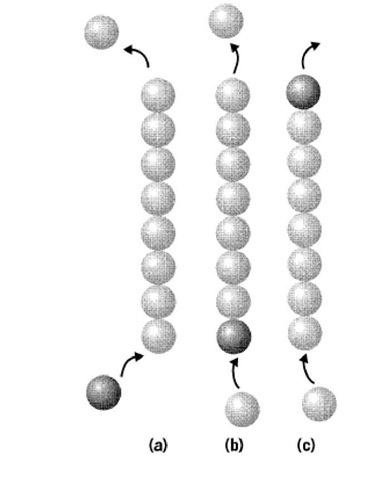
Figure 1. In a polymer where subunits add preferentially at one end (bottom, a) and depolymerize from the opposite end (top, a), a treadmilling phenomenon will exist, with a flux of subunits passing through a polymer that maintains a constant length. In (b) a "labeled" subunit adds at the bottom, and this subunit would travel the length of the polymer before exiting, as in (c).