Tissue culture was originally understood to mean the maintenance and, in some cases, growth in vitro of small explanted fragments of tissue from an animal or plant, (1, 2), but it has become a generic term covering a wide range of in vitro cultivation techniques. Cell culture, where the tissue is dissociated into individual cells, which may be propagated adherent to glass or plastic, or in suspension, is the technique most widely used. Organ culture, where the tissue is not disaggregated, but maintained at the air-liquid interface as a complete fragment, is a method that retains the architecture of the tissue, but does not allow propagation. Maintenance of small fragments of tissue at the solid-liquid interface, that is, attached to the glass or plastic substrate (primary explant culture), permits the outgrowth of cells on to the substrate by migration and cell proliferation, and can give rise to a propagated cell line.
Tissue culture, defined in its generic sense, is used when it is important to regulate the physical and physiological microenvironment of the cells, maintain a consistent, validated stock of characterized cells, and/or minimize the use of laboratory animals. It also allows the introduction of exogenous genomic DNA with a high efficiency (see Transfection), the study of signaling mechanisms under controlled conditions with a purified population of cells, the measurement of cytotoxic and genotoxic responses, and the production of proteins with appropriate post-translational modifications (see Protein Engineering). Tissue culture, however, will not mimic in vivo conditions accurately, because of the difference in the cellular microenvironment, the absence of metabolizing enzymes normally found in the liver, and the difficulty of reproducing the fully differentiated cell phenotype in vitro. Continuous cell lines are also genetically unstable and often genotypically heterogeneous.
1. History
Opinions vary as to when tissue culture actually commenced, as there was a gradual transition from the earlier studies of pathologists with isolated fragments of tissue to the demonstration of actual growth (3). Tissue culture implies that the explanted tissue or cells can be maintained for more than a few hours and will display some aspect of growth. The first success in this area is usually attributed to Harrison (1), who was able to maintain spinal cord from frog embryo in a natural medium of clotted frog lymph for up to 4 weeks and observed outgrowth of nerve fibers from the explant. Others were able to repeat Harrison’s observations and made attempts to passage the resulting cultures (4). Propagated cell lines (see Cell Line) were derived from the outgrowth of several types of tissue, but it was not until after World War II that extensive use was made of trypsin to passage cells, producing a single-cell suspension that could be divided accurately and even cloned (5).
Numerous attempts were made to generate new cell lines (6), assisted by the development of antibiotics, which were incorporated into culture media to prolong culture life without contamination. Subsequently, the development of laminar flow cabinets in the late 1960s and early 1970s provided a protected environment, facilitating aseptic technique without the necessity of continued culture in antibiotics. Around this same period, it was observed that cells could be stored frozen in culture medium, with a preservative added, for extended periods (7-9), and this gave further protection against loss by contamination or incubator failure. This led to a rapid proliferation of cell lines, and it was not until Gartler (10) initiated studies with enzyme polymorphisms, which could distinguish some cell lines from others by the isoenzymes characteristic of the original tissue, that a more serious contamination problem was discovered, namely, cross-contamination. This was confirmed by karyotype analysis (11), and by the mid-1970s, it was estimated that around 30% of all cell lines in current use in North America were cross-infected with HeLa Cells (12). Many of these, such as KB, Hep-2, Girardi heart, and Chang liver, are still in current use, often not identified by the user as HeLa-contaminated. This problem has been recognized by the major cell banks, who now label stocks suspected as carrying HeLa markers (13).
The continued use of antibiotics, combined with natural products, such as serum and trypsin, brought in another crisis when Barile (14) and others reported that large numbers of propagated cell lines were contaminated with mycoplasma, too small to detect readily without special staining, and not always as catastrophic to the culture as are most bacterial infections. These microorganisms were too small to be filtered out by the sterilization procedures of the day and, once contaminating a cell line, were very difficult to remove. The development of a fluorescent staining technique (15) and other assays for mycoplasma detection, together with a reduced porosity of sterilization filters used in serum manufacture, means that contamination is readily detected, and further contamination preventable, by constant screening and use of materials from reputable sources. Nevertheless, mycoplasma contamination, introduced from infected cell lines, primary cultures, or the operator, is still widespread and requires constant vigilance to eliminate.
Modern tissue culture, carried out in high grade facilities with ultrapure water and media components and proper validation and authentication procedures, is now a highly reproducible technique employed in academic research laboratories, diagnostic laboratories, and industrial-scale production of biopharmaceuticals.
2. Types of Culture
2.1. Cell Culture
This is the maintenance and propagation of cells as a uniform monolayer or suspension (16). A cell culture may be derived from a tissue by three main methods of primary culture.
1. Primary explant technique. A small fragment of tissue is placed on the base of a culture vessel so that it adheres, either spontaneously, facilitated by scratching the plastic surface, trapping the tissue under a coverslip, or use of surface tension or clotted plasma. A primary culture arises by outgrowth from the explant across the surface of the dish.
2. Mechanical disaggregation. The tissue may be disaggregated by chopping with scalpels or scissors or by forcing the tissue through a mesh screen or syringe needle. The resultant suspension of cells and small fragments is allowed to settle and form an adherent cell monolayer on a glass substrate or the correct grade of plastic (see below).
3. Enzymatic disaggregation. Tissue may be disaggregated by incubation in a number of different proteinases; trypsin, collagenase, and Dispase are the most common. Trypsin requires the absence of serum, which contains trypsin inhibitors, but collagenase and Dispase are insensitive to proteinase inhibitors in serum. As for mechanical disaggregation, the resultant cell suspension, washed free of enzyme by centrifugation and resuspension, is allowed to settle to the base of the dish to form an adherent monolayer (17).
The outgrowth from a primary explant culture, or the monolayer generated from mechanically or enzymatically disaggregated cells, may be subcultured (see Cell Line) and transferred to fresh culture vessels (17). This process, also known as passage , is usually achieved by treating the monolayer with trypsin to dissociate the cells from each other and the substrate, resuspending the cells in fresh medium with serum (to inhibit the residual trypsin), and diluting into fresh culture vessels (17). After the first subculture the culture becomes a cell line (see Cell Line).
3. Organ Culture
Organ cultures are not as widely used as cell cultures, as they require considerably more effort to initiate and cannot subsequently be propagated. They do, however, provide a means to retain at least some of the histological structure of the tissue, and with it some of its phenotypic characteristics. Organ cultures can be maintained for up to 3 weeks (although longer periods have been recorded) and may contain areas of localized cell division, but growth is limited. Tissue, or preferably a whole organ, from the embryo survives better, and may show some net growth in culture, but the growth and cell survival are limited by the dimensions of the tissue explant. Under normal conditions (20% oxygen at atmospheric pressure), the radius of a spherical organ culture is limited to a maximum of 500 |im. The organ culture may extend in shape in two dimensions, as long as one dimension remains at 500 |im or less. The inward diffusion of oxygen, and the outward diffusion of CO2, are optimized by growing the tissue at the air-liquid interface; access to the nutrient medium is provided by a permeable support, usually a porous membrane on a stainless steel mesh grid. Organ cultures have been found particularly useful for cultivation of skin (18), fetal bone (20), and various embryonic organs during organogenesis (21).
4. Histotypic Culture
It is possible to create the cell-cell interactions provided by tissue-like densities, while still having the convenience and reproducibility of cell lines, by growing cell cultures to high cell densities. This can be achieved in several ways: (1) By growing cells in a filter well insert, where the cells are crowded, but adequate medium is provided by access to a relatively large reservoir (22); (2) growing cells as stirred aggregates, called spheroids; generated by growing the cells at high concentrations on agar or agarose in a multiwell plate, so that the cells form aggregates in the bottom of the meniscus generated by the agar in the well—these are then stirred at low speed in suspension; similar aggregates may be generated by growing cells in suspension in zero gravity in a slowly rotating chamber (23); and (3) Growing cells on the outer surface of perfused microcapillary bundles, where the cells are seeded in the outer chamber holding the hollow fibers, and medium is pumped through the fibers from a reservoir (24).
5. Organotypic Culture
Histotypic culture can be adapted to cocultivation of two, or more, different cell types, in an attempt to simulate heterotypic cell interactions, in addition to the homotypic cell interactions achieved in histotypic culture. Skin culture has become a classic example, where epidermal epithelium maintained in coculture with dermal fibroblasts embedded in collagen will generate well-differentiated keratinocytes expressing involucrin, filaggrin, and cross-linked keratin (25). The requirement for collagen is similar to many specialized cultures, which require elements of the extracellular matrix, such as collagen, laminin, and fibronectin, for proliferation and differentiation.
6. Monolayer and Suspension Cultures
Most normal cells attach to plastic and require to spread out on the plastic before cell division will commence (see Contact Inhibition). Attachment is mediated by transmembrane integrin receptors (see Cell Adhesion Molecules), which bind components of the extracellular matrix deposited on the substrate by the cells. Hence, coating the substrate with matrix molecules, such as fibronectin or collagen (26), or conditioning the substrate by previously culturing cells on it and removing them with detergent in water (27), can often facilitate cell attachment and subsequent proliferation.
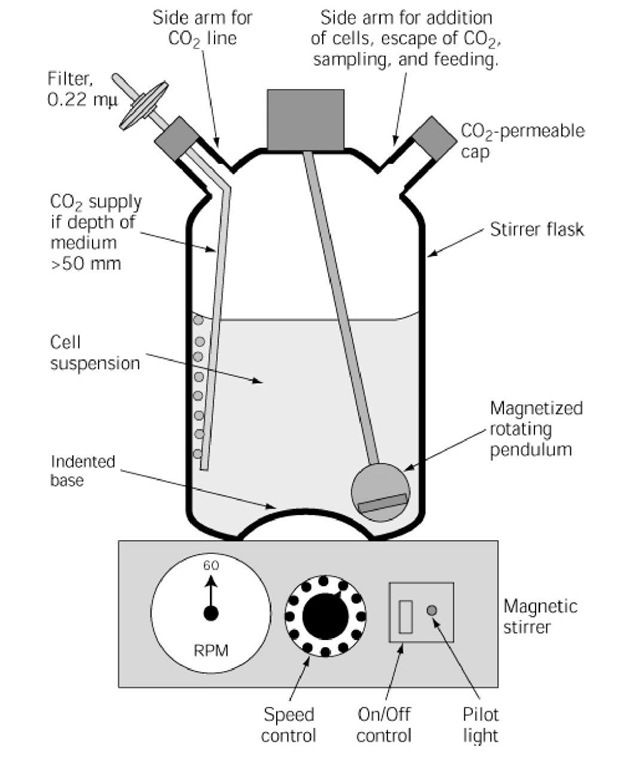
Some cells will grow readily in suspension, either spontaneously (eg, murine ascites tumors) or by mechanical stirring (eg, HeLa^) (Fig. 1). These cells are said to be anchorage independent, and the cultures are usually derived from the transformed cells of a tumor or from normal cells that have transformed in vitro, either spontaneously or following mutagenesis (see Neoplastic Transformation). Suspension cultures can be propagated in large bulk without elaborate mechanisms for increasing the surface area. Gas exchange is improved when the depth of medium exceeds 50 mm by sparging the medium with a mixture of 5% CO2 in air.
Figure 1. Cross-sectional diagram of a stirrer vessel on a magnetic stirrer. The side arm at top right is used to add cell suspension and collect samples; it has a permeable cap to allow escape of CO2. Filtered CO2 in air is supplied via a filter to the side arm at top left; it is required if the depth of the culture medium exceeds 50 mm. Agitation is achieved by a magnet, enclosed in a glass pendulum, and is driven by the magnetic stirrer at ~60 rpm.
Suspension cultures can be scaled up easily, deliver a large bulk of cells at one time, and do not need trypsin for harvesting the cells from the culture. They can also be maintained in a steady state of growth by regulating the rate at which medium is added to balance the rate of cell proliferation and by withdrawal of surplus cells. Such cultures are called biostats or vivostats and enable the concentrations of cells, nutrients, and products, and the pH, osmolality, and gas tension, to be kept constant over prolonged periods (28).
7. Culture Vessels
Cell cultures are usually grown in disposable plastic flasks, petri dishes, or multiwell plates, that have been treated with plasma discharge, or some similar process, to create a net negative charge on the surface of the flask. Flasks are preferable for long-term propagation, dishes for cloning or where direct access to the growth surface is required (and are cheaper), and multiwell plates for replicate sampling. Where a large number of cells is required (>1 * 109), roller bottles, or multisurface propagators are required for attached cells, and large fermentors for suspension-grown cells.