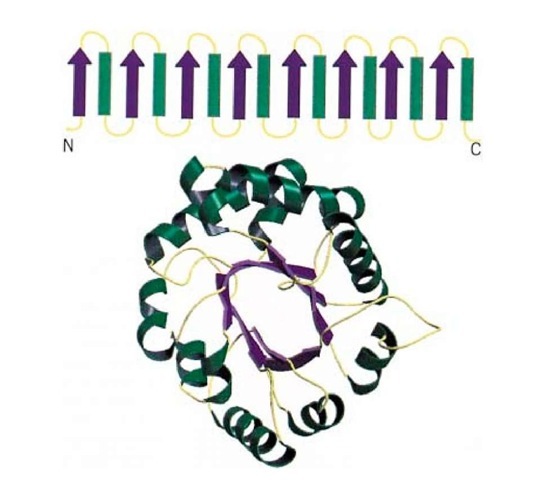
The TIM barrel is named after the protein triose phosphate isomerase, or TIM, in which this type of domain of a protein structure was first observed. The TIM barrel has a central cylinder or barrel of b-sheet formed from eight parallel b-strands, with strands 1 and 8 hydrogen bonded to each other to close the b-barrel. Each b-strand is connected to the next by a linking a-helix; the eight a-helices form a concentric layer surrounding the central parallel b-barrel. Both the strands and helices of the TIM barrel have a pronounced right-hand twist (Fig. 1). The topology of the TIM barrel gives rise to its other names, the (ba) 8 or (a/b)8 barrel.
Figure 1. A TIM barrel. (Top) Schematic representation of the topology of the TIM barrel, with b-strands depicted as purple arrows and a-helices depicted as green cylinders. The connecting regions between the helices and cylinders are shown in yellow, and the N- and C-terminal ends of the motif are labeled. (Bottom) Schematic representation of the backbone of triose phosphate isomerase (1) showing a typical TIM barrel structure. The center of the barrel is formed from the b-strands forming a parallel b-sheet (shown as purple arrows), and the outer layer is formed from a-helices (shown as green coils) that connect the b-strands. The connecting regions between helices and strands are shown in yellow.
This very common fold is found in many proteins with diverse functions and no detectable sequence identity, so their similar structures are thought to be an example of convergent evolution. Although the activities and sequences of these proteins vary, their active sites are all formed by loop regions at the carboxyl ends of the b-strands that connect to the a-helices. The particular enzymatic activity of the enzyme is dependent on the amino acid residues in these regions. The TIM barrel forms one of two major classes of a/b domains, and the other class is typified by the nucleotide-binding motif.