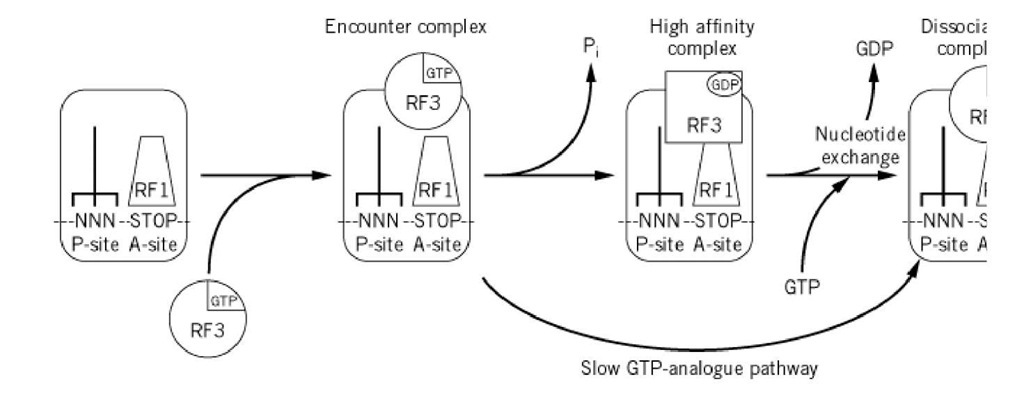
Most textbooks end the description of protein biosynthesis with the release-factor-mediated release of the completed polypeptide chain from the peptidyl- transfer RNA. This is a gross oversight, because there is an additional crucial step in protein synthesis: recycling of ribosomes through dissociation of the termination complex (1). In bacteria, this process requires a ribosome-recycling factor (RRF, originally called ribosome-releasing factor). This process must be fundamental, because the gene for RRF is essential for cell growth, and the living cell must reuse the ribosome, release factors, and tRNA for the next round of protein synthesis. Upon release of the polypeptide chain, the ribosomal P and A sites remain occupied with a deacylated tRNA and a tRNA-mimicking release factor, respectively. A translocase is probably required to forward deacylated tRNA and release factor to the E and P sites of the ribosome, respectively. It is speculated that RF-3 or EF-G may catalyze this final translocation reaction. Alternatively, this post-release ribosomal complex may be dissociated directly by the action of RRF and RF-3 or EF-G (Fig. 1). Bacterial release factor RF-3 accelerates the dissociation of RF-1 and RF-2 from the ribosome in a GTP-dependent manner, and fast recycling of ribosomes requires both RF-3 and RRF (2).
Figure 1. An RF-3 recycle factor model (2). This explains how RF-3 accelerates the dissociation of RF-1 / 2 from the rib tRNA.