Tautomers are structural isomers that interconvert rapidly (1), so they will all exist in a solution of the compound at equilibrium at ambient temperature. The concept of tautomerism was introduced by Laar (2). In most cases, tautomers are generated by the structural and electronic rearrangements induced by moving a single proton (3). In the reaction catalyzed by triosephosphate isomerase, the substrate, glyceraldehyde-3-phosphate, the enediol intermediate, and the product, dihydroxyacetone-phosphate, comprise two tautomeric pairs. As shown in Figure 1, glyceraldehyde-3-phosphate and the enediol are structural isomers that result when a proton is transferred from C2 to the aldehyde oxygen, as indicated by the dashed arrow. Transfer of the proton is accompanied by a rearrangement of the electrons, as indicated by the solid curved arrows. Similarly, dihydroxyacetone-phosphate and the enediol intermediate are tautomers, as a single-proton transfer also permits their interconversion. Because of the imprecision of the phrase "rapidly," the property of being tautomers may not be transitive. For example, glyceraldehyde-3-phosphate and dihydroxyacetone-phosphate are not considered to be tautomers, although they are both tautomers of the enediol intermediate.
Figure 1. Two examples of keto-enol tautomerization. A proton from the a-methylene of either glyceraldehyde-3-phosp] dihydroxyacetone-phosphate is transferred to the carbonyl; after the depicted electron rearrangement, a structural isomer
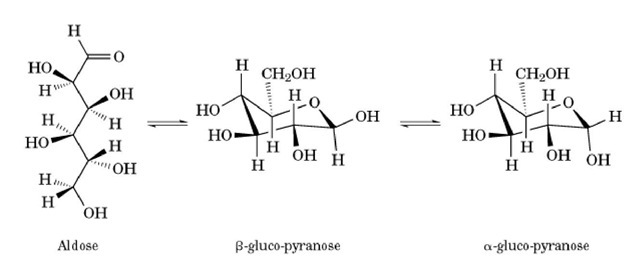
A second form of tautomerism important in biochemistry is the existence of both open-chain and ring forms of monosaccharides. Thus, the three common forms of glucose shown in Figure 2 are all tautomers, because they will interconvert rapidly in aqueous solution. The two cyclic pyranose forms are not only tautomers, but also epimers and diastereomers.
Figure 2. The three tautomeric forms of D-glucose. These are rapidly interconverting structural isomers. Note that the tautomeric forms differ by more than the rearrangement of a proton, consistent with Ingolds expanded definition (1).
The existence of tautomers can be detected spectroscopically, in that all the tautomeric forms may give rise to different spectral features. For glucose, it is possible to observe 1H-NMR resonances from all three tautomeric forms. It is also possible to monitor their "rapid" equilibration in aqueous solution by following the difference in optical rotation after dissolving in water a pure crystal of either of the pyranose forms.
The equilibrium constant between tautomeric forms often is very far from 1, but the less favored tautomer may be the biologically active form. In nucleic acid bases, keto-enol tautomerism can change the hydrogen bond acceptor carbonyl to a hydrogen bond donor hydroxyl (4). Tautomeric forms of all four nucleic acid bases exist, but the rare tautomer occurs less than 0.01% of the time (5).
![Two examples of keto-enol tautomerization. A proton from the a-methylene of either glyceraldehyde-3-phosp] dihydroxyacetone-phosphate is transferred to the carbonyl; after the depicted electron rearrangement, a structural isomer Two examples of keto-enol tautomerization. A proton from the a-methylene of either glyceraldehyde-3-phosp] dihydroxyacetone-phosphate is transferred to the carbonyl; after the depicted electron rearrangement, a structural isomer](http://what-when-how.com/wp-content/uploads/2011/05/tmp8144_thumb1_thumb.jpg)