Biotransformations that involve sulfate conjugation were first described more than 120 years ago. Originally, sulfation was connected mainly with drug inactivation and metabolism, which is initiated by an oxidative reaction that inactivates the target (phase I), followed by subsequent conjugation of, for example, sulfate or glutathione (phase II). Such conjugation increases the drug’s water solubility and thereby facilitates excretion of the end product. Since then, many additional targets and functions of sulfate conjugation has been revealed: (i) inactivation of hormones and catecholamines, (ii) activation and inactivation of xenobiotics, (iii) removal of endogenous products (eg, bile salts), and (iv) modulation of protein structure and function.
The sulfation reactions are catalyzed by members of a family of enzymes designated sulfotransferases (ST). They use 3′-phosphoadenosine 5′-phosphosulfate (PAPS) as a co-substrate and sulfate donor. Thus, sulfation denotes conjugation of a given substrate with a sulfuryl group (-SO3-); accordingly, the reaction should more correctly be designated as sulfonation. The functional groups of the affected substrates are typically either hydroxyl groups, resulting in formation of an ester, or unprotonated amines, leading to sulfamates.
Sulfation is widespread in nature, and sulfotransferases are found in both plants and animals. In mammals, sulfation appears to be ubiquitous in all tissues, but the type of sulfation and the target specificity may well be tissue-specific. The targets range from various xenobiotics, neurotransmitters, steroid hormones, and thyroid hormones, to proteins, glycoproteins, and glycosaminoglycans. The targets are localized both in the cytosol and in the protein secretion pathway (Fig. 1). These intracellular localizations define two subsets of sulfotransferases: (i) the soluble sulfotransferases in the cytosol and (ii) the membrane-associated sulfotransferases in the Golgi apparatus. Along with their differences in enzymatic properties and substrate specificities, each subset will be described according to their intracellular localization.
Figure 1. Schematic diagram of the intracellular localization of sulfation reactions and targets in a typical mammalian cell. In the cytosol, brackets indicate whether sulfation leads to activation (+) or inactivation (-). An overview of PAPS synthesis and localization is also shown at the bottom. Xenobiotics refer to exogenic products administered to the cell, such as drugs.
1. PAPS Biosynthesis
The co-substrate PAPS is produced in the cytosol in a two-step enzymatic reaction from ATP and inorganic sulfate (Fig. 1). For sulfation reactions in the Golgi, PAPS is translocated to the lumen of the Golgi by a specific translocase (1). The rate-limiting step in PAPS formation is the formation of APS, and its steady-state concentrations are relatively low and display tissue-specific variations. Even in the liver, where PAPS concentrations are highest, stores can be depleted within minutes during maximal sulfation rates. Under normal conditions, PAPS concentrations are independent of the availability of inorganic sulfate. In rat liver, however, challenge of the conjugation reaction with high doses of exogenous substrate decreases the PAPS concentrations and depletes sulfate in the serum. This is in contrast to mice, in which the sulfotransferase activity limits the sulfation rate under corresponding conditions (2). Thus, sulfate availability, which changes with age and during physiological changes and diseases, can be affected by drug intake. Such depletion of sulfate stores can introduce serious toxic effects. Hence, regulation of PAPS synthesis is equally important for sulfation, as is the regulation of expression of the sulfotransferases.
2. Sulfation in the Cytosol
Sulfation in the cytosol involves a number of different functions and targets. The reactions have been studied both by endocrinologists and pharmacologists, and enzymes have been named after the substrate used for investigation. The overlapping substrate specificities has led to a confusing nomenclature of the enzymes, which is being unraveled by the cloning of the genes for the sulfotransferases. At present, five cytosolic sulfotransferases have been characterized in humans, but more will probably be identified in coming years (see Table 1).
Table 1. Overview of the Best Characterized Human Cytosolic Sulfotransferases a
|
Enzyme |
Endogenous Substrate |
Xenobiotic Substrates |
|
TS PST1 (P-PST) |
Iodothyronine, estrogen |
Hydroxyarylamines, simple phenols |
|
TS PST2 (SULT1A2) |
Unknown |
Hydroxyarylamines, simple phenols |
|
TL PST (M-PST) |
Catecholamines, iodothyronine |
1-Naphthol, minoxidil, salbutamol |
|
EST |
Estrogen |
Ethinylastradiol, equilenin |
|
DHEAST (HST) Androgens, cholesterol, bile salts, estrogen |
Aliphatic alcohols, benzylic alcohols |
|
a The most common names of the enzymes are given in abbreviations: TS PST-1 (-2), thermostable phenol ST-1 (-2); P-PST, phenol-preferring phenol ST; SULT1A2, sulfotransferase 1A2; TL PST, thermolabile phenol sulfotransferase; M-PST, mono-amine preferring phenol ST; EST, estrogen ST; DHEAST, dehydroepiandrosterone ST; HST, hydroxysteroid ST.
Sulfation in the cytosol has a number of endogenous targets. For example, sulfation plays a role in the inactivation of thyroid hormones. The prohormone thyroxine (T4) is converted into T3, the active component, in peripheral tissue. Inactivation occurs by deiodination or by sulfation by thermostable phenol sulfotransferase 1 (TS PST1). Sulfated T3 does not bind to the thyroid receptor, and sulfated T3 and T4 are rapidly degraded. Similarly, sulfation of catecholamines such as dopamine by TL PST (thermolabile phenol sulfotransferase) renders dopamine inactive as a neurotransmitter. In fact, about 90% of the dopamine circulating in plasma is found as sulfate conjugates, but the exact role of this derivative is at present unclear.
In addition, sulfation plays a key role in homeostasis and metabolism of another class of important hormones: the steroids. Sulfation of steroids excludes binding of the steroids to their receptors and thereby eliminates their effect. However, steroid sulfation may also be a way of transporting more soluble steroids, and the biological activity can be regained by the aid of tissue-specific sulfatases leading to specific receptor binding with subsequent signaling. The most abundant steroid in plasma is sulfated dehydroepiandrosterone (DHEA). The sulfated form of DHEA is metabolized 100-fold more slowly than nonsulfated DHEA, but the biological significance of its high plasma concentrations, which decrease with age, is not yet understood. However, DHEA serves as a precursor of both estrogen and androgen. DHEA is the target of DHEA sulfotransferase, which is highly abundant in the liver. This enzyme is also responsible for the sulfation of cholesterol and secondary bile salts. Secondary bile salts are generated from salts that are secreted by the liver and by bacteria in the intestine. These salts have a toxic effect on hepatocytes, but sulfation facilitates the excretion of the bile salts through the urine.
Sulfation also plays an important role in the biochemistry of estrogen. Sulfated DHEA is the major precursor of estrogens and androgens. Also, sulfated estrone is the most abundant estrogen in the plasma. Like other steroid hormones, estrogens are inactivated by sulfation. Hence, sulfotransferases serve a regulatory role of estrogen activity. For example, estrogen sulfotransferase is expressed in a cyclic manner in the endometrium and is induced by progesterone. Thus, this sulfotransferase may regulate stimulation by estrogen during the menstrual cycle. However, three sulfotransferases are capable of sulfating estrogens, albeit with different affinities, and this makes characterization of the reactions difficult.
Besides inactivation, sulfation can activate a number of compounds. One example is minoxidil, an antihypertensive agent, which is active only after endogenous sulfation. However, sulfation is also engaged in a less fortunate type of bioactivation: generation of mutagens. Many carcinogens, including dietary procarcinogens such as safrole and estragole, only obtain their mutagenic properties after metabolic modification, such as by sulfation. This effect of sulfation was first demonstrated for hydroxamic acids and hydroxylamines, but at present the list of affected compounds is long, and most cytosolic sulfotransferases are involved in the process.
In recent years, much has been learned about the cytosolic transferases and their activities. Recently, the first three-dimensional protein structure of estrogen sulfotransferase was presented (3), and new sulfotransferase variants will probably be identified. The availability of sulfotransferases as recombinant proteins will also facilitate future studies of the bioactivity of the sulfotransferases.
3. Sulfation in the Golgi
In the Golgi, two classes of molecules are sulfated: proteins, which are sulfated on tyrosine residues, and glycosaminoglycans. The types of sulfotransferases involved are different and do not overlap in substrate specificity.
Sulfation of tyrosine residues was first discovered as a modification of fibrinogen in the mid-1950s. In the 1960s, the modification was found in a few peptide hormones, but only in the beginning of the 1980s was the truly widespread nature of tyrosine sulfation recognized (4). Since then, sulfation of a number of proteins has been demonstrated. Tyrosine sulfation occurs in the trans Golgi network; as a consequence, the proteins affected are secretory proteins, membrane proteins, and presumably lysosomal proteins (5). The modification is the most common side-chain modification of tyrosine residues, and up to 1% of the tyrosine in a given cell may be sulfated. Tyrosine sulfation appears to be ubiquitous in all tissues and has been traced back to Caenorhabditis elegans.
Tyrosine sulfate appears to be involved in various types of protein-protein interactions. The following effects are well established: (i) Tyrosine sulfation of the peptide hormone cholecystokinin is a prerequisite for receptor binding; (ii) sulfation of the leech anticoagulant protein hirudin increases the affinity toward thrombin; (iii) sulfation increases the affinity between factor VIII and von Willebrand factor, (iv) sulfation is necessary for the binding of P-selectin to P-selectin glycoprotein ligand 1; (v) sulfation increases the intracellular processing of the peptide hormone gastrin; and (vi) sulfation alters the secretion kinetics of Drosophila yolk protein 2. For many of the proteins that are known to be sulfated (eg, the b-amyloid precursor protein), the function is unknown. This is in part due to the difficulties in prediction of tyrosine sulfation sites. Furthermore, the high frequency of tyrosine sulfation suggests that more proteins than those known today will be found to be sulfated. Based on the structures surrounding known tyrosine sulfation sites, and in vitro experiments using synthetic peptides, a consensus sequence for prediction of sulfation has been suggested. The main feature is the presence of an acidic residue on the amino-terminal side of the tyrosine and additional acidic residues neighboring the sulfation site. At least partial sulfation can be obtained, however, without an acidic residue on the amino-terminal side of the tyrosine residue, as found in the peptide hormone gastrin. It is at present unclear how common partial sulfation is and what are the precise structural requirements for sulfation. With the recent identification and cloning of two tyrosylprotein sulfotransferases [TPST-1 (6) and TPST-2 (7, 8)], new tools are available for precise definition of the requirements. It is also possible that additional TPST family members will be identified.
The two TPST complementary DNAs each encode a type II integral membrane protein that displays an overall identity of 64% in amino acid sequence. Both enzymes are glycoproteins, of 370 and 377 amino acid residues, respectively, and contain a short (7-residue) cytosolic tail that is probably involved in intracellular transport and localization of the enzymes. Little is known about the regulation of the enzymes, but, with the recent cloning, this will be a topic for further investigation.
Another class of molecules sulfated in the Golgi is the glycosaminoglycans (GAGs). These molecules are disaccharides and constituents of the proteoglycans, proteins that are also glycosylated to an extreme degree, ending up with molecular weights of up to several million. The proteoglycans consists of a complex protein core of varying composition conjugated with between 1 and 100 GAG chains. There are seven classical GAGs, six of which are sulfated in at least one position: chondroitin-4-sulfate, chondroitin-6-sulfate, dermatan sulfate, keratan sulfate, heparin, and heparan sulfate. Accordingly, large proteoglycans can contain a high number of negatively charged groups. These charges are central for the physiological functions of glycosaminoglycans and proteoglycans in interacting with other proteins, similar to the biological role of tyrosine sulfation. Glycosaminoglycans were traditionally considered structural components of the extracellular matrix in connective tissue. However, recent years have disclosed much more active roles of glycosaminoglycans.
The best-characterized effect of sulfation is in the anticoagulant effect of heparin. Heparin binds antithrombin III in a reaction that is strictly dependent on sulfation of a specific position of heparin and alters the conformations of both molecules. Heparin also modulates the effect of basic fibroblast growth factor (FGF), and the receptor binding of this growth factor is affected by heparan sulfate. Recent studies suggest that selection between FGF-1 and FGF-2 by the activating proteoglycan during development is mediated by changes in heparan sulfate side chains, and possibly by a change in sulfation pattern during differentiation and growth arrest (9). Similarly, the chondroitin sulfates, which are involved in cell adhesion, cell migration, and possibly also neural development, show differences in sulfation pattern during development and tumor progression that are likely to influence the physiological function of the proteoglycans carrying these sulfates. Considering the increased interest in the functions of proteoglycans in signal transduction and other types of cell signaling, along with the developmental changes in compositions of glycosaminoglycans, additional examples of functional significance of sulfate in proteoglycans can be expected.
4. Homology of Sulfotransferases
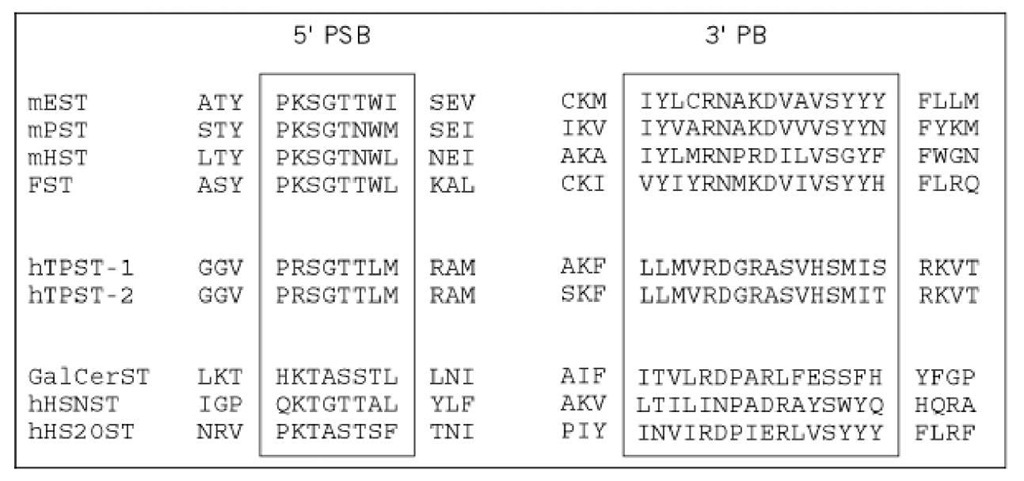
The sulfotransferases with all the different targets described here have surprisingly little homology, suggesting that these enzymes may have evolved independently. This was disputed recently, when homology between two protein regions involved in PAPS binding from cytosolic transferases and from several glucosaminoglycan/heparan sulfotransferases was demonstrated (10). Following the cloning of the genes for the two TPSTs, a general comparison of all the types of sulfotransferases is possible. This shows that the TPSTs differ from the other membrane-associated sulfotransferases and from the cytosolic enzymes, even in the most conserved regions (Fig. 2). Hence, if the sulfotransferase have evolved from a common ancestor, they have diverged sufficiently to have very different primary structures.
Figure 2. Alignment of partial protein sequences of sulfotransferases. Two spatially separated regions that are important for PAPS binding are the primary homologous regions of sulfotransferases. They are denoted the PSB loop and the 3′-binding site. All sulfotransferases were aligned as described (8) except for the TPSTs. The abbreviations of the protein sequences are: mEST, mouse estrogen sulfotransferase; mPST, mouse phenol sulfotransferase; mHST, mouse hydroxysteroid sulfotransferase; FST, flavonol sulfotransferase (plants); hTPST, human tyrosylprotein sulfotransferase; GalCerST, human 3′-phosphoadenylsulfate galactosylceramide sulfotransferase; hHSNST, human heparan sulfate A-deacetylase/A-sulfotransferase; hHS2OST, human heparan sulfate 2-sulfotransferase.