On amino acid starvation, bacterial cells exhibit an adaptive response, known as the stringent response, for repression of production of the machinery for cell growth, such as the translation and transcription apparatus (1, 2). The requirement for amino acids is, however, relaxed by mutations in a single RNA control locus called relA (3). The search for a regulatory molecule under the control of relA led to the discovery of accumulation under starved conditions of unusual guanine nucleotides, called magic spots I and II (4), which were later identified as ppGpp and pppGpp, respectively (5, 6).
1. Synthesis and Degradation of ppGpp
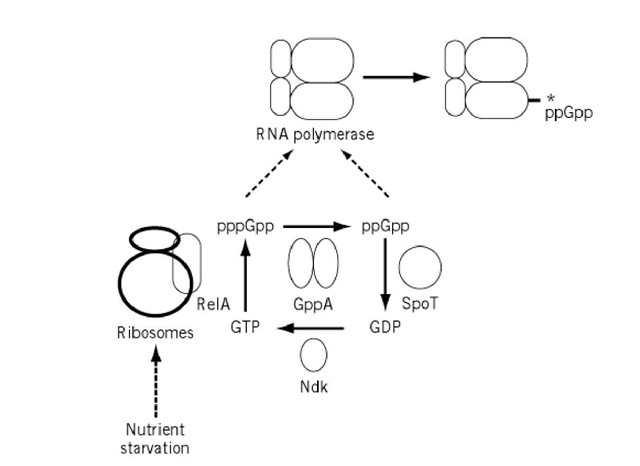
The relAl gene encodes the RelA enzyme (p)ppGpp synthetase I, originally called stringent factor, that catalyzes the synthesis of pppGpp and ppGpp (7-9) [Fig. 1]. This RelA protein is associated with ribosomes, but it can be released by high-salt washes. The (p)ppGpp synthesis reaction requires that the ribosomes have bound messenger RNA and codon-specified uncharged transfer RNA bound at the acceptor (A) site (10, 11). Thus, the synthesis of (p)ppGpp takes place at an elongation step in protein biosynthesis without ribosomal movement (10). The purified RelA enzyme, free of ribosomes, is virtually inactive, but it can be activated by inducing conformational changes with methanol and detergents.
Figure 1. Regulatory circuit of the stringent response. Ribosome-associated RelA catalyzes the synthesis of both pppGpp and ppGpp from GTP and GDP, respectively, but, from the high Km value, GTP is considered to be the natural substrate. The conversion of pppGpp to ppGpp is catalyzed by several different enzymes but, from its low Km value, the gppG gene product is considered to play a major role in vivo. SpoT catalyzes the breakdown of (p)ppGpp to GTP or GDP; in the presence of high concentrations of GTP or GDP, however, it acts as (p)ppGpp synthetase II. The most probable target of (p)ppGpp action is the b subunit of RNA polymerase.
The synthetic reaction of (p)ppGpp is a pyrophosphoryl group transfer from ATP to the 3′ hydroxyl of GDP or GTP acceptor nucleotides (7, 12). The high Km (Michaelis constant) values for GTP and GDP (0.5 mM) are within the physiological concentration range for GTP but not for GDP, suggesting that pppGpp is the most likely primary product in vivo (7, 9). Conversion of pppGpp to ppGpp can be catalyzed by a variety of enzymes in vitro, including two exopolyphosphatases, products of the gppG andppx genes (13-15) [Fig. 1].
The spoT gene encodes an enzyme for ppGpp degradation, ie, removal of the 3′-pyrophosphate residue from either ppGpp or pppGpp (16) [Fig. 1]. The protein SpoT is also associated with ribosomes but is active even after release by high-salt treatment (17, 18). The spoT operon includes five genes in the order: gmk (guanylate kinase), rpoZ ( RNA polymerase w subunit), spoT (ppGpp 3′-pyrophosphatase), spoU (RNA 2′-O-methyltransferase) and recG (junction-specific RNA helicase) (19). At least three gene products are involved in guanine nucleotide metabolism. RNA polymerase-associated w protein was suggested to control the ppGpp sensitivity of RNA polymerase (20), but this concept has been challenged (21). SpoT catalyzes the relA–independent synthesis of (p) ppGpp, the reverse reaction of (p)ppGpp degradation and, therefore, SpoT is now recognized as (p) ppGpp synthetase II (22, 23).
2. Target of ppGpp Action
The concept that the target of ppGpp action is RNA polymerase is proposed on the basis of observations that in vitro transcription of stringently controlled genes by purified RNA polymerase is inhibited by ppGpp in the absence of additional factors, even though the step of transcription affected by ppGpp seems to vary, depending on which template is used (24-28). Early in vitro studies, however, gave conflicting results regarding the effect of ppGpp on transcription of the stringently controlled genes. This disagreement has now been attributed to various factors, including the heterogeneity in RNA polymerase preparations used in early studies, the involvement of several additional factors for maximum transcription of the stringent promoters, the presence of multiple promoters with different specificities in the same and different genes, nonlinear DNA dependency of the stringent promoters, and inhibition by ppGpp of not only initiation but also transcription elongation.
In parallel with in vitro transcription studies, genetic studies have reached the same conclusion. Some rifampicin -resistant rpoB mutants of an Escherichia coli B relA parental strain were found to be hypersensitive to ppGpp levels (29). Among point mutations of the b subunit of RNA polymerase generated by the suppression of amber mutations in the rpoB gene, three amino acid substitutions were found to result in a relaxed RNA-control phenotype (30). This result has been confirmed by in vitro transcription studies using RNA polymerase purified from these rpoB mutants with relaxed phenotype (31).
The outcome of these biochemical and genetic observations supported the simple model that ppGpp is one of the transcription factors that interacts with RNA polymerase and modulates its promoter selectivity (32-34). Evidence for direct contact between ppGpp and the RNA polymerase b subunit has accumulated [Fig. 1]. Owens et al. (35) demonstrated crosslinking of azido-ppGp to RNA polymerase. Reddy et al (36) showed stoichiometric binding of a fluorescence-labeled ppGpp to RNA polymerase and estimated its distance from the rifampicin-binding domain of RNA polymerase. Chatterji et al (37) synthesized azido-ppGpp and, using a radiolabeled derivative, identified the sites of ppGpp crosslinking on the b subunit of RNA polymerase, which are close to those of rpoB mutations conferring relaxed phenotype (31). Spontaneous missense suppressor alleles, which are able to confer complete prototrophy to E. coli mutants lacking both of the (p) ppGpp synthases, RelA and SpoT, were localized in RNA polymerase subunit genes, 97% in rpoB and rpoC and 3% in rpoD. Mutations in conserved region 3 of sigma factor s increases the ppGpp sensitivity of RNA polymerase (38). One possibility is that these mutant s induce conformational changes of the b subunit, leading to alteration in its affinity for ppGpp.
3. Inhibition of RNA Synthesis by ppGpp
Escherichia coli possesses seven ribosomal RNA (rRNA), rrn operons, which all contain a tRNA gene in the spacer region between the 16S and 23S rRNA genes and the 5S rRNA gene, in the region distal to the 23S gene. The promoter region of these long transcripts is sufficient to determine their regulation during the stringent response, as well as during growth-rate control. Two promoters have been identified, upstream P1 and downstream P2, which together display complex transcriptional regulation. Upstream of the P1 promoter are regions implicated in transcript activation, ie, the UP element (or prokaryotic enhancer) and the Fis protein-binding site (39). Activity of the rrn promoters in vitro is strongly dependent on DNA supercoiling in purified assays (40). The C-terminal domain of RNA polymerase alpha-subunit is involved in recognition of both the DNA UP element and the Fis protein (33, 34). From the three-dimensional structure of this domain (41), the same protein surface seems to be involved in contact with both the DNA and protein factors, each leading to modulation of the promoter-recognition properties of RNA polymerase. Direct measurement of P1 and P2 activities in vivo indicated that the strong promoter P1 plays a major role in rRNA synthesis in rapidly growing cells and is subject to inhibition during the stringent response, but that P2, a weaker constitutive promoter, is insensitive to amino acid starvation (42, 43). Using an in vitro mixed transcription system, Kajitani and Ishihama (25) revealed that the upstream P1 promoter was specifically repressed by ppGpp, whereas the downstream P2 promoter was virtually unaffected.
Several lines of evidence indicate that transcription of stringently controlled genes, including tRNA genes and some ribosomal protein genes, by purified RNA polymerase holoenzyme Es is also inhibited in the presence of ppGpp (25, 44). Taken together, these observations indicate that these stringently controlled genes share a common regulatory DNA sequence that is specifically recognized by ppGpp-associated RNA polymerase.
4. Stringent Signal
Sequence comparisons suggested the presence between the -10 Pribnow Box and the +1 RNA transcription start site of a GC-rich region that has been called a "discriminator" and has been proposed as a common feature of all promoters negatively regulated during the stringent response (32, 45). When the GC-rich discriminator of the tyrT gene was replaced with an AT-rich sequence, the promoter became more active than the wild-type one. The mutant promoter also became resistant to negative stringent control in vivo (44).
Transcripts of the tufB operon contain four tRNA genes upstream of the tufB gene and display negative stringent control in vivo and ppGpp inhibition in vitro (46). Studies of ppGpp inhibition of promoter mutants implicated the critical role of base-pair positions -7 to -4, which normally have the sequence GCGC (47). Changing each of the GC pairs at the -7 to -4 positions individually to AT pairs led to a reduction in the degree of ppGpp inhibition in vitro. The upstream and strong rrn promoter P1 is responsible for both stringent control and growth-rate-dependent control, whereas the activity of P2 is weak and is involved in a low level of constitutive expression of the rrn genes. At slow growth rates, the P2 activity predominates and is responsible for the persistence of rRNA synthesis, because it is resistant to ppGpp inhibition. The core promoter region of P1, from nucleotides -41 to +1, is sufficient for growth-rate-dependent control (48), which overlaps the discriminator signal for stringent control.
5. Transcription Activation by ppGpp
Venetianer (49, 50) observed that the his operon mRNA was abundant during the stringent response. This finding suggested that the expression of certain genes, including those of amino acid biosynthetic operons, is activated during the stringent response. It is currently thought that many genes are subject to positive ppGpp control. Two-Dimensional Gel Electrophoresis analysis of proteins synthesized during the stringent response reveals that the fraction of proteins showing positive control is approximately equal to those showing inhibited synthesis (51, 52).
As with negative control, ppGpp has been implicated as the regulatory signal mediating positive control. The relA gene product is required for maximal expression in vivo of the his operon (53, 54). Again, these effects are promoter-specific, for they occur even when the his attenuator is deleted (54). Positive regulation of his operon expression has been verified as occurring at the promoter (55, 56). Enhancement of transcription of the arg and trp operons in vitro was also found in the presence of ppGpp (25, 57).
6. Role of ppGpp in Growth Control
Early studies suggested that, during steady-state growth, both relaxed and stringent strains displayed an inverse correlation between basal ppGpp levels and both growth rate and RNA accumulation levels (58, 59). The relationship between ppGpp levels and rRNA accumulation during very slow steady-state growth is, however, complicated by a number of features: (1) rRNA is synthesized but is not assembled into ribosomes, as a result of unbalanced synthesis of ribosomal proteins and/or rapid degradation of newly synthesized rRNA (59-61) and (2) ppGpp affects the expression of many genes in different ways, either repression or activation, and to various extents. Thus, the hierarchy of gene expression is markedly influenced, affecting directly or indirectly the rate of rRNA synthesis.
Growth-rate control is observed both in the presence and in the absence of ppGpp (39) suggesting that ppGpp does not play a major role in growth-rate control. Instead, Gaal et al. (62) provided conclusive evidence that the concentration of substrate nucleoside triphosphates determines the growth-rate-dependent control of rRNA transcription. Thus, the core promoter sequence may specify the concentration of nucleoside triphosphates required for efficient initiation of transcription.
Although ppGpp is not directly involved in growth-rate-dependent control, it is involved in growth-phase control by inducing the synthesis of RNA polymerase ss subunit for transcription of stationary phase-specific or stress response-specific genes (63). In the stationary phase of E. coli growth, ppGpp also induces the production of polyphosphate (64), which ultimately leads to alteration in the gene expression pattern by binding to the RNA polymerase and modulating its promoter selectivity (65).