Streptomycin (SM) was the first aminoglycoside antibiotic agent, introduced in 1944 by Schatz, Bugie, and Waksman (1). It has been used as an effective antimicrobial substance for the treatment of infectious diseases caused by aerobic Gram-negative and Gram-positive organisms, especially as a first-line drug for the therapy for tuberculosis. However, the appearance of resistant microorganisms (2), SM’s ototoxicity, and the introduction of the less toxic broad-spectrum b-lactam antibiotics decreased SM’s clinical usefulness in recent years. It has also been used as a tool for analysis of the mechanism of translation during protein biosynthesis, because it binds to the 30 S subunit of ribosomes and causes misreading of the messenger RNA.
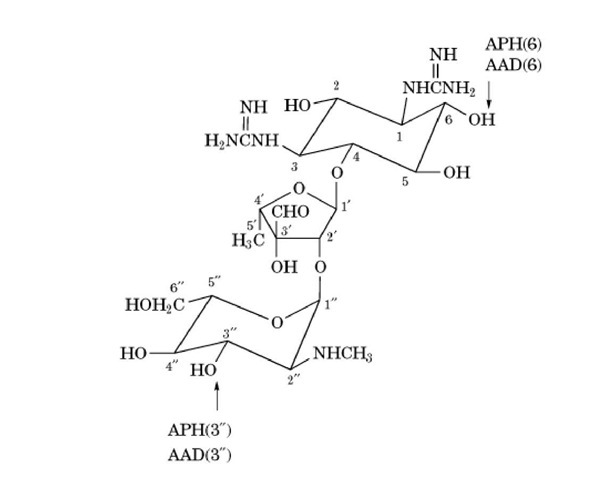
SM was discovered from culture supernatants of Streptomyces griseus, although it is now known also to be produced by S. bikiniensis, S. olivaceus, S. mashuensis, S. galbus, S. rameus, and S. glovisporus streptomicine. SM is one of the aminoglycosidic aminocyclitols and belongs to the oligosaccharide group of basic water-soluble antibiotics (Fig. 1). It consists of streptidine, L-streptose, and #-methyl-L-glucosamine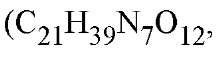 molecular weight 581.58) (3).
molecular weight 581.58) (3).
Figure 1. The structure of streptomycin. The sites that are modified by inactivating enzymes are indicated. Key: APH, ^-phosphotransferase; AAD, O-adenyltransferase.
1. Mechanism of Action
SM exhibits bactericidal activity (killing bacteria) by inhibiting prokaryotic protein biosynthesis, although most such inhibitors have only bacteriostatic activity, ie, inhibiting bacteria’s growth but not killing them. Its bactericidal activity can be explained by four processes: (i) SM associates with the cell surface by ionic binding and diffuses through the outer membrane into the periplasmic space, in an energy-independent process (4, 5). This diffusion process may be owing to a "self-promoted uptake" mechanism, in which SM displaces divalent cations that cross-bridge adjacent lipopolysaccharide molecules, permeabilizing the outer membrane (6); (ii) SM enters the cytoplasm, using the electron transport system to cross the inner membrane (energy-dependent phase I) (6); (iii) SM binds to the 30 S ribosomal subunit at nucleotides 911-915 of the 16 S ribosomal RNA in Escherichia coli (7, 8). The 900 stem and the 530 stem/loop regions of the 16 S rRNA interact with ribosomal proteins S4 and S12 (9-12). Proteins S4, S5, and S12 have been reported to be close neighbors in the protein map of the 30 S ribosomal subunit (13), and these proteins and their associated RNA segments constitute an area of the 30 S ribosomal subunit known as the accuracy region (13). SM binds irreversibly to the 30 S ribosomal subunit and subsequently forms abnormal initiation complexes, so-called streptomycin monosomes, by fixing the 30 S/50 S ribosomal complex at the start codon of the mRNA (14). Accumulation of the abnormal initiation complex blocks further translation of the mRNA, elicits premature termination, and incorporates incorrect amino acids, producing abnormal proteins; and (iv) The abnormal proteins are incorporated into the cell membrane, and this alters its permeability. The alteration of the cell membrane stimulates the further entry of SM through the energy-dependent phase II process (15) and causes its bactericidal activity (16).
The energy-dependent transport across the inner membrane is inhibited by divalent cations (eg, Ca and Mg ) and by acidic and anaerobic conditions (6). Therefore, SM shows a reduced level of bactericidal activity in the presence of Ca and Mg , or in acidic conditions, and does not have bactericidal activity against anaerobic organisms and facultative bacteria cultured anaerobically (17). The bactericidal activity of SM competes with other inhibitors of protein bio-synthesis, such as chloramphenicol, erythromycin, and tetracycline, but not with puromycin (18).
It has been reported that organisms with mutations at the rpsL gene, encoding the S12 protein, exhibit SM-resistant or SM-dependent phenotypes. Furthermore, mutations in the genes encoding the S4 or S5 protein—ram (ribosomal ambiguity) mutations—of an SM-dependent mutant that cannot grow in the absence of SM cause it to revert to SM independence and to grow in the absence of SM (19).
2. Resistance
Two resistance mechanisms to SM have been reported. One is the modification of SM by modifying enzymes, and the other is mutation of the genes encoding the target sites (20, 21).
The modifying enzymes are usually encoded by resistance plasmids, but in Serratia marcescens and Providencia sturartii, they are encoded by chromosomal genes (22). Two modifying enzymes have been elucidated: O-phophotransferase, encoded by the aph gene, which phosphorylates at the 3”-OH or 6-OH groups of SM, and O-adenyltransferase, encoded by the aad gene, which adenylates at the 3”-OH or 6-OH groups (Figure 1). Cross resistance between SM and kanamycin has been reported. However, because kanamycin, neomycin, paromomycin, and gentamicin are not affected by these modifying enzymes, SM-resistant strains possessing the modifying enzymes are sensitive to these aminoglycosides. Resistance plasmids encoding the modifying enzymes have been found in Enterobacteriaceae, Pseudomonas aeruginosae, and Staphylococcus, but not in mycobacteria.
The other resistance mechanism is the alteration of target sites. This occurs in the S12 protein (the rpsL gene) and in the region of the 16 S rRNA (the rrs gene) that interacts with the S12 protein. In E. coli, substitution mutations on the rpsL gene are replacement of Lys43 by arginine, isoleucine, or asparagine and replacement of Lys88 by arginine (23). Similar mutations of the rpsL gene are found in mycobacteria (24-30), with Lys43 replacement by arginine or threonine and Lys88 replacement by arginine or glutamine. In addition, Arg9 replacement by histidine and Val93 replacement by methionine have been reported. Resistance mutations in the rrs gene have occurred in the regions of the 900 stem and the 530 stem/loop, which interact with the S12 protein as described in the text above: C^T transition mutations at positions 491, 512, or 516, A^C or T transversion mutations at position 513, C^A or G transversions at position 903, or A^G transition at position 904. Meyer et al (26) have shown that approximately 30% of resistant M. tuberculosis strains have missense mutations at Lys43 or Lys88 of the S12 protein. They have also reported that the resistance level to SM is dependent on the mutation site; mutations at Lys43 or Lys88 of the S12 protein confer high-level resistance, whereas mutations at the 530 stem/loop of 16 S rRNA give intermediate-level resistance. In mycobacteria, approximately 80% of SM-resistant strains carry mutations in either the rpsL or the rrs gene (28-30), but low-level resistant strains possess no such mutations (25, 26). It has been suggested that alteration of the permeability of the cell wall is a third resistance mechanism, because the addition of the membrane-active detergent Tween 80 lowered the resistance level (26). Resistance mutations in S12 protein and 16 S rRNA are phenotypically recessive. Therefore, mutation of a single gene cannot generate the resistant phenotype in organisms that have multiple copies of the rrs gene, such as E. coli (with seven copies) and M. smegmatis (with two). Because the slowly growing mycobacteria, eg, M. tuberculosis and M. leprae, have a single rRNA gene copy (31, 32), mutation of the rrs gene can confer resistant-phenotype organisms.
SM-dependent mutant strains that require SM for growth possess mutations of the S12 protein: Pro42 altered to leucine, Lys43 altered to glutamic acid, deletion of Lys88, Pro91 altered to leucine or arginine, Gly92 altered to aspartic acid, and deletion of Arg94 (23); plus a mutation of the S4 protein (the rpsD gene). Furthermore, a revertant mutation from SM dependence to SM independence has been reported for Gln73 (to proline) in S4 protein (33).
3. Clinical Uses
SM is active against mycobacteria and a number of aerobic Gram-negative rods (Enterobacteriaceae) and Gram-positive cocci (Enterococcuceae and Staphylococcus ) (2). It is not active, however, against Streptococcus pyogenes, S. pneumoniae, fungi, and anaerobic bacteria. SM is now used rarely except for the treatment of tuberculosis and some unusual infections, for example, tularemia (Francisella tularensis), plague (Yersiniapestis), and Weil’s disease (Leptospira interrogans serovar. icterohaemorrhagiae). With the emergence of multiple drug-resistant (MDR) strains ofM. tuberculosis, SM has been renewed as the first-choice drug for tuberculosis. However, SM cannot enter into living cells, so it cannot kill and eradicate intracellular microbes such as mycobacteria.
As SM is a polar cation, it is poorly absorbed from the gastrointestinal tract. It is primarily excreted via the kidneys, essentially unchanged. Therefore, oral administration cannot be expected to be effective against systemic infection.
Considerable intrinsic toxicity, mainly in the form of nephrotoxicity and vestibular or auditory toxicity, is a characteristic of all the aminoglycosides. The toxicities are caused by damage to the eighth cranial nerve and to hair cells in the cochlea. This side effect is irreversible, even after discontinuance of the drug. The nephrotoxic potential varies among the aminoglycosides and is usually reversible when the drug is discontinued.