Two of the major challenges facing all life forms are propagation of the species and survival under adverse conditions. One mechanism used by many organisms to meet these challenges is to encapsulate their genome in a specialized cell structure, known as a spore, that is metabolically inactive and often highly resistant to environmental assault. The spore protects the organism’s genome until an appropriate time when the instructions encoded on the DNA can be used to produce a new individual. Because the morphogenic process of spore formation (sporulation) is often associated with extracellular production in many prokaryotic species of commercially valuable commodities (antibiotics, enzymes, bioinsecticides, and more) and is related to pathogenicity and toxicity of others, spore research remains an active and important scientific area. Additionally, sporulation cycles in some organisms serve as relatively simple examples of cellular differentiation events that can be analyzed at the molecular level.
Spores can be grouped into two broad types that are not necessarily mutually exclusive: reproductive spores and resting spores. The former, made by various plant species and some fungi, can be either single-celled or multicellular. Produced in enormous amounts and passively disseminated by wind, water, or animals after detachment from the parental organism, reproductive spores can each give rise to a new individual and thus serve for rapid increases in the population of the species. Resting, or dormant, spores are produced by many prokaryotic species (notably in the genera Bacillus, Clostridium, Streptomyces, Myxococcus) and some lower eukaryotes (eg, Dictyostelium) as a survival mechanism when the organism encounters an unfavorable environment. These spores can remain in a quiescent state until favorable growth conditions return, at which time they undergo a differentiation process, known as germination and outgrowth, to regenerate actively growing and dividing cells (vegetative cells). There is good evidence that dormant bacterial spores can remain viable for up to a century (1), and some recent observations suggest that survival for millions of years may also be possible (2). Although considerable progress continues to be made in unraveling the details of spore formation in Myxococcus, Streptomyces and Dictyostelium (for reviews see Refs. 3-5), the molecular biology of sporulation has been best characterized for Bacillus subtilis.
Extensive research on the structure and properties of Bacillus spores has served as a paradigm in the field. The two most important factors responsible for the longevity of these bacterial spores are:
1. encasement of inactivated enzymes, ribosomes, and DNA in a dehydrated central core surrounded by a multilayered protective shell; and
2. protection of the DNA present in the core from environmental damage.
The factors responsible for the inactivity of spore enzymes are not entirely understood, but the lowered water content of the core (about 5- to 10-fold less than that present in the cytoplasm of growing cells) is undoubtedly a major contributor to both this property and to overall heat resistance (6). In spores of Bacillus and related genera, a class of fascinating proteins, known as a/b SASP (small acid- soluble proteins), coat the spore DNA and protect it from damaging assaults caused by desiccation, heat, oxidation and ultraviolet radiation (7). The multilayered integument (8) surrounding the core provides a permeability barrier preventing access of some damaging agents and functions to maintain physicochemical integrity of the core itself. From the inside out, the protective layers of a mature Bacillus spore generally include:
1. a cytoplasmic membrane enveloping the core;
2. a thin layer of peptidoglycan known as the germ cell wall that is very similar, or identical, to vegetative cell peptidoglycan;
3. a thick layer called the cortex that is comprised of a different form of peptidoglycan;
4. a second membrane;
5. a thick layer of proteinaceous coat that itself can be subdivided into layers of distinct appearance; and
6. an outer membranous layer known as the exosporium.
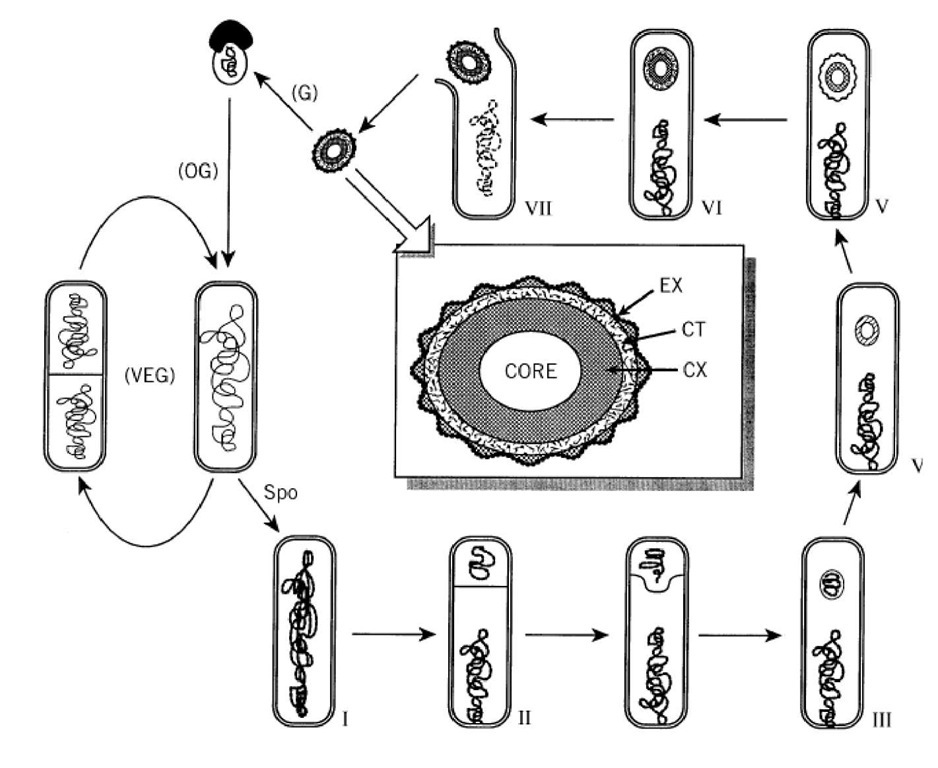
The morphological landmarks of B. subtilis sporulation are illustrated schematically in Figure 1. Mutation analysis has identified over 100 genes required for normal spore formation. Many of these genes are designated by a specialized nomenclature indicating at which morphological stage mutations in the gene halt development. For example, spoil mutants arrest at stage II (asymmetric septum formation) and do not proceed to stage III (forespore engulfment). Different operons are distinguished by a single capital letter (spollA, spollB, etc.), and different cistrons within the same operon are denoted by additional letters (spollAA, spollAB, etc.). Not all sporulation-associated genes use this nomenclature and, in many instances, elucidation of the biochemical function of the gene product has led to renaming of the gene (eg, spollAC was discovered to encode a s factor of RNA polymerase, and the gene is now called sigF).
Figure 1. Sporulation of Bacillus subtilis. Landmark morphological stages are: I, axial filament of chromosome; II, polar septation dividing the sporangium into smaller forespore and larger mother cell compartments; III, engulfment; IV, corte formation; V, coat formation; VI, maturation; VII, lysis of mother cell and release of spore. Under laboratory conditions 37%, the process takes about 8 h. Germination (G), outgrowth (OG), and vegetative growth (VEG) complete the life cyc The center inset is a diagram illustrating major features of a mature spore. The layers are not drawn to strict scale; spore membranes and distinctions in the coat layer are not shown. CX, cortex; CT, protein coat; EX, exosporium.
Sporulation is but one of the developmental options available to B. subtilis cells when they are deprived of nutrients and enter a stationary phase of growth. Numerous interconnected regulatory circuits sense the exact environmental and metabolic conditions and dictate which option is chosen. Initiation of the sporulation pathway is absolutely dependent upon the level of phosphate flux through a signal transduction system known as the phosphorelay (9). The phosphorelay is a complex assemblage of protein kinases, protein phosphatases and phosphorylatable proteins, with each component apparently being a focal point for input of signals indicative of nutritional and physiological status. There is only sketchy evidence suggesting how the activities of the kinases are regulated (10, 11) and how intracellular GTP levels might affect the phosphoprotein phosphotransferase encoded by spoOB (12). Most current excitement concerns the signaling roles played by small peptides that affect the activity of the phosphatases (13-15). The signals, whatever their exact nature, regulate the level of the ultimate output of the phosphorelay: SpoOA protein in its phosphorylated form (Spo0A~P04). Spo0A~P04 is a transcriptional regulatory factor that is responsible for activating expression of three operons (spoIIA, spoIIG, spoIIE) required for transition from stage II to stage III of sporulation. Additionally, Spo0A~P04 represses transcription of the abrB gene, and this leads to expression of many functions that had been kept silent during vegetative growth due to the regulatory actions of AbrB protein (16). Spo0A~P04 is also required for the hallmark of stage II: formation of an asymmetrically-positioned division septum. Although the exact mechanistic role of Spo0A~P04 in this event is unclear, it is clear that commitment to sporulate occurs soon after formation of this septum (17). Up until this point, the sporulation pathway could have been aborted and the cell channeled either back into vegetative growth or else into an alternative developmental state of stationary phase.
Completion of the sporulation septum divides the cell (now termed the sporangium) into two compartments: the forespore (prespore) and the mother cell. [Because the mother cell subsequently engulfs the forespore compartment (stage III) and further development and maturation occurs in this configuration, the spore produced is more properly called an endospore.] Each compartment proceeds to express different sets of genes necessary for endospore formation. This compartmentalization is primarily due to differential activity of alternative RNA polymerase sigma factors (18), but it is likely that the differences in chromosome condensation in each compartment also play an important role (19). A fascinating aspect of sigma factor compartmentalization is that intercompartmental communication mechanisms temporally link the activity occurring in one compartment to the differential activity that occurs within the other. The general scheme of this crisscross regulation (20) is that s activity in the forespore leads to activation of s in the mother cell; s activity in the mother cell is necessary for s activation in the forespore; s -directed transcription in the forespore is required for s activity to follow s activity in the mother cell. Much remains to be elucidated concerning how these complex interplays occur, but many of the details now known provide illustrative examples as to how cellular morphology can affect gene expression, and vice versa.
Spo0A~P04-activated expression of the operon ( spoIIA) encoding sF occurs prior to completion of the sporulation septum, and so all three protein products of the operon (SpoIIAA, SpoIIAB, s ) are present in both compartments of the sporangium. However, s activity occurs only in the forespore due to a regulatory mechanism termed "partner switching" (21). The SpoIIAB protein is an anti- sigma factor that can bind s and sequester it in an inactive form. SpoIIAB can also bind SpoIIAA (an anti-anti-s factor), and its relative affinity for each mutually exclusive partner is influenced by the intracompartmental ATP/ADP ratio (see Adenylate Charge). Additionally, SpoIIAB is a kinase able to phosphorylate SpoIIAA, and SpoIIAA~P04 cannot bind SpoIIAB (22). Although many questions remain unanswered, the current model is that in the forespore the ATP/ADP ratio is low; this condition favors binding of SpoIIAB-SpoIIAA and release of free, active s . Furthermore, a membrane-associated phosphatase encoded by spoIIE (whose transcription is activated by Spo0A~P04) becomes localized to the septum but becomes active only on the forespore side, thus counteracting SpoIIAB kinase action upon SpoIIAA in that compartment. In the mother cell, SpoIIAB-s F binding predominates, due apparently due to a higher ATP/ADP ratio, SpoIIAB phosphorylation of SpoIIAA, and absence of SpoIIE phosphatase activity (23).
Two genes expressed in the forespore due to s F-directed transcription are sigG and spoIIR. Gene sigG encodes the s factor responsible for late forespore gene expression, and spoIIR encodes a protein necessary for s activation in the mother cell. As in the case of spoIIA, the operon (spoIIG) encoding s is expressed prior to septum completion as a result of Spo0A~P04-mediated transcriptional activation. The initial proteins produced are a membrane-bound putative proteinase
(SpoIIGA) and an inactive precursor of s (pro-s ). The production of SpoIIR in the forespore compartment is the signal responsible for initial processing of pro-s to s in the mother cell (24).
SpoIIR is secreted out of the forespore, and this process somehow activates SpoIIGA-dependent proteinase activity on the mother cell side of the septal membrane (25). It is not clear whether SpoIIR provides an absolute directionality that prevents pro-s to s processing from occurring in the forespore or if additional mechanisms that selectively inactivate sE in that compartment also exist (26).
Relatively little is currently understood concerning regulation of s activation in the forespore, but it is apparent that sE-controlled transcription of the eight-cistron spolllA operon in the mother cell is a crucial event (27). It also is clear that s activity in the forespore is required for s activity to appear in the mother cell. Synthesis and activation of sK involves a number of mechanisms, including one that could occur only in a terminally differentiated cell destined to lyse. An intact, contiguous gene (sigK) encoding sK is not normally present on the chromosome: The sigK open reading frame is interrupted by a 48-kb element referred to as skin. During sporulation, sE and a transcriptional regulatory protein, SpoIIID, activate transcription of a gene (spolVCA) in the skin element. The SpoIVCA protein is a site-specific recombinase that catalyzes excision of a circular DNA molecule in which the truncated portions of sigK become fused in-frame (see Translation). The resultant intact copy of sigK is transcribed by s /SpoIIID, and the messenger RNA is translated to produce an inactive pro-sK protein. This precursor is then processed to active sK by a proteinase whose activity is dependent upon s -directed expression of genes in the forespore (28). Once activated in their respective compartments, both s and s direct further transcription of their own genes via positive autoregulatory feedback loops and are responsible for expression of gene products necessary for the final steps of endospore development and maturation.
The cascade of s factors during B. subtilis sporulation is obviously a primary means of achieving temporally regulated expression of genes required for the ordered assembly of the morphological structure of the endospore. Yet many details concerning this process remain to be elucidated, and it seems highly probable that other, perhaps novel, mechanisms await discovery. Although many genes responsible for the physical components and assembly of the spore structures (eg, the peptidoglycan cortex, protein coat) have been identified, very little is actually known about the biochemistry and enzymology underlying these assemblages. Additionally, much work remains to be done in order to understand the process of spore germination and outgrowth, an area that has received far too little attention in the past. Nevertheless, the continuing rapid pace of discovery has shown that sporulation is an excellent experimental system for examining fundamental aspects of cellular differentiation.