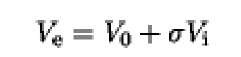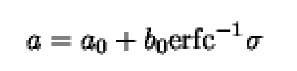Molecular biologists have recently become increasingly interested in purification and biochemical analysis of gene products that function in a broad range of biological systems. Size exclusion chromatography (SEC) provides a method for both analytical and preparative-scale separations of biological macromolecules . Historically, SEC has been referred to as gel filtration chromatography when applied to the separation of proteins in aqueous (native) conditions (1). The most common uses of SEC are for determination of the size of macromolecules and for their purification. A less appreciated, but very powerful, application of the method is for the analysis of the interactions of macromolecules, to determine their stoichiometries and energetics. In SEC, solutes are separated on the basis of their size. In the case of proteins, the molecular size or hydrodynamic volume is usually specified in terms of the Stokes Radius(1). SEC differs from other types of chromatography, such as ion-exchange or affinity chromatography, in that the analytes are not separated on the basis of their differential interaction with the column resin. In fact, an inherent assumption of gel filtration chromatography is that there are no interactions between the protein solute and the stationary phase. This entry provides descriptions of the SEC technique and of methods of analysis of SEC data.
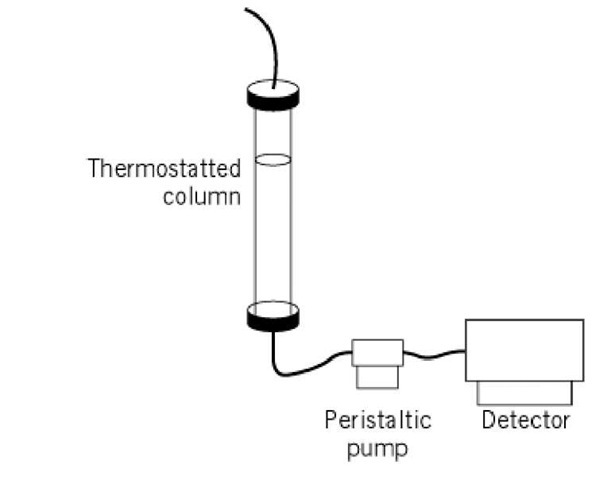
The basic experimental apparatus for standard SEC is shown in Figure 1 (2). The size exclusion resin is packed into a column, which may be jacketed to maintain temperature via connection to a circulating water bath. Detailed discussion of commercially available resins is provided in molecular sieve resins. Protein samples to be analyzed by SEC are loaded onto the top of the resin as either a small or large zone (see discussion below), and allowed to enter the resin at a flow rate determined by a pump connected at the column outlet. Once a sufficient amount of sample has entered the column, the sample is replaced by column buffer, and transport of the macromolecules proceeds. The eluted proteins can be detected most conveniently using an in-line detection system, most commonly a UV-visible absorbance detector. However, fluorescence, or even in-line scintillation counting of radioactivity, can also measure the protein eluted. Alternatively, the eluted material can be characterized after its elution and collection, using a very wide variety of techniques. Although standard low pressure chromatographic equipment has traditionally been used for SEC, the use of FPLC and HPLC systems has become increasingly popular for performing small-zone measurements (3). The utility of HPLC for large-zone experiments, however, has been rigorously demonstrated in only one case (4).
Figure 1. Schematic diagram of a standard low pressure gel filtration chromatography experimental setup. For a discussion of the experimental details, the reader is referred to Reference 2.
1. Zonal Analysis
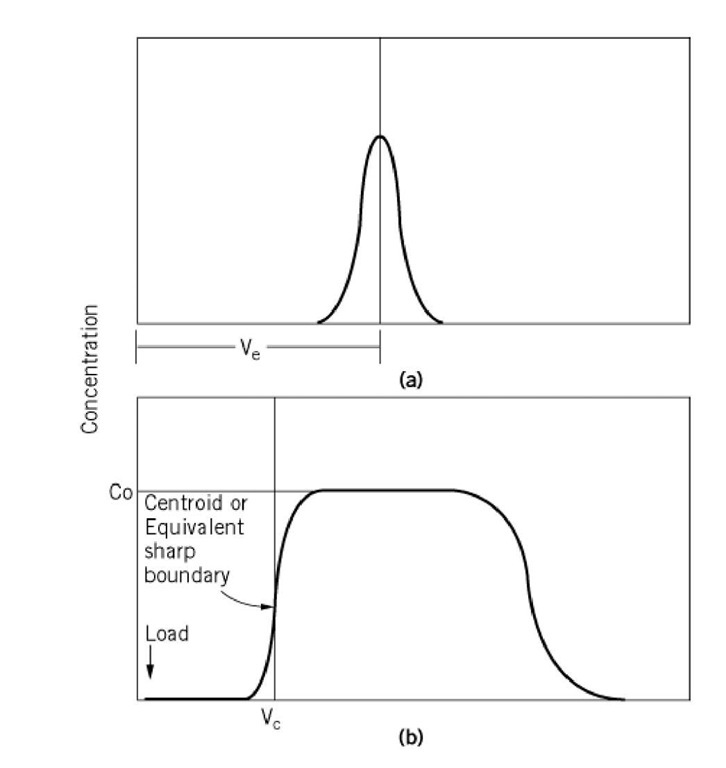
The most common type of SEC experiment involves loading a small volume of solute onto the column. This is referred to as a small-zone experiment (Fig. 2a) or zonal analysis, and it may be used for either analytical or preparative separations. The volume of the sample used in this type of experiment is approximately 1-4% of the total column bed volume (1). As the sample flows through the column, its transport is accompanied by spreading of the zone as a result of diffusion. A single homogeneous peak detected at the exit port of the column should have a Gaussian shape, the apex of which defines the elution volume of the solute. Applying a mixture of solutes with different elution properties will result in multiple Gaussian-shaped peaks.
Figure 2. Schematic representations of results of (a) small-zone and (b) large-zone size exclusion chromatography: Ve—the elution volume of the small zone, Co—the initial concentration of the loaded sample in the large-zone experiment, Vc—the centroid or equivalent sharp boundary of the leading edge of the large zone. This last term is analogous to the elution volume measured in the small-zone experiment.
2. Boundary Analysis of Large Zones
A second type of SEC measurement involves chromatographing a large volume of solute on the resin. This type of experiment, which is referred to as large-zone gel filtration chromatography, is especially useful for measuring protein-protein interactions (5, 6). One advantage of this technique over small-zone experiments is that dilution of the sample does not have to be considered. As a small zone of protein is transported through a column, the zone is diluted, so the concentration at the column outlet can be as much as 100-fold lower than that of the loaded sample. If dilution of the protein results in its dissociation, the oligomeric state of the protein at the point of elution may differ significantly from that of the loaded sample. This makes it impossible to interpret the results of small-zone experiments in terms of protein assembly equilibrium. In a large-zone experiment, in contrast, a sufficiently large volume of protein is loaded onto the column so that the edges of the zone, leading and trailing, are fed by a plateau of protein at the constant initial loading concentration (Fig. 2b). The elution volume for a large zone is determined from the centroid or equivalent sharp boundary of the leading or trailing edge of the zone. This elution position reflects the average size of the protein at its initial loading concentration. If a protein dissociates, its average size, as determined from its elution volume, will, according to mass action, decrease with zones loaded at decreasing protein concentrations. The data relating the measured partition coefficient to protein concentration are analyzed using appropriate mathematical models, to obtain the stoichiometry and energetics of the protein assembly process (6).
The information obtained from large-zone SEC experiments is thus analogous to that obtained from sedimentation equilibrium centrifugation measurements. The advantages of the SEC technique over centrifugation are that the instrumentation is of only moderate cost, and consequently available to a larger number of laboratories, and that the method can be more readily applied to study of very tight macromolecular associations. This has been demonstrated in studies of the lambda phage cI repressor, for which dimerization equilibrium constants in the nanomolar range of concentration were measured by large-zone SEC (7). An excellent discussion of the application of large-zone analytical gel filtration chromatography for measurement of protein-protein interactions is provided in Reference 6.
3. Size Measurements by SEC
In order to obtain size information about a solute from an SEC experiment, the column must first be characterized in terms of the volumes accessible to analytes. The volume of an SEC column is divisible into three parts: (1) the volume external to the packing material, Vq, ( 2) the volume contained within the porous resin beads that is accessible to small molecules, V-; and (3) the volume occupied by the packing material itself, Vg (1, 5). The values of V0 and V-x are determined experimentally by measuring the elution volumes of, respectively, a large solute that is totally excluded from the interior of the resin and a small solute that has access to all pores of the resin. The elution volume, V of a solute that is partially included in the pores of the gel filtration resin can be related to the void and internal volumes of a column by the following equation:
where s is the partition coefficient of the solute or the fraction of the internal volume of the resin, V-v that is accessible to the analyte. A detailed discussion that relates these measured volumes to the microscopic properties of the sieving resin is contained in molecular sieve resins.
It is the partition coefficient, s, that, when compared with the values measured for proteins of known size, provides information about the molecular size of an unknown macromolecule. If a series of proteins of known size or Stokes radius are subjected to SEC, a linear relationship between partition coefficient and size is observed (5). For proteins that are well approximated as hydrated spheres, a linear relationship between the elution volume and molecular weight will be observed (1, 5). One approach to analysis of SEC data to obtain information about the size of the protein was developed by Ackers (8). This relationship assumes that penetrable volumes within column packing material are distributed randomly with respect to the size of the protein that can penetrate them. The Stokes radius, a, for a protein in A units (1.0 x 10-10) is related to the inverse error function complement (erfc-1) of s as
where a 0 and ^ are calibration constants for a column and are obtained by chromatographing a series of proteins of known size or Stokes radius. Values of the inverse error function complement are compiled in tables provided by the National Institute of Standards and Technology (NIST), or they may be readily calculated (9). The Stokes radius for a protein obtained from SEC measurements will accurately reflect the radius of a spherically shaped molecule, but not all proteins are well modeled as spheres, approaches to deal with other shapes are given in Stokes Radius.