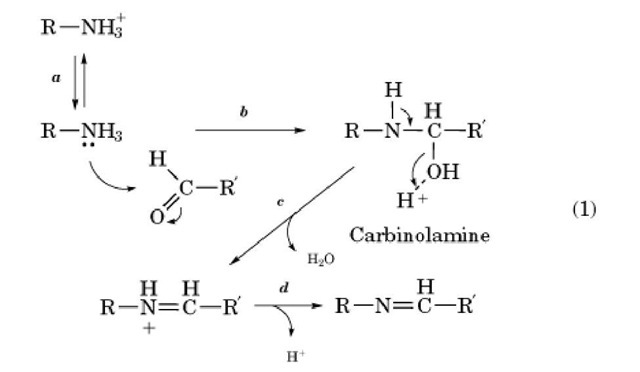
Schiff bases (also known as Schiffs bases, imines, or azomethines) are unsaturated compounds formed by the condensation of amino groups with aldehydes or ketones (1-4): (Eq. 1)
Sell iff base
The amine will be reactive in its un-ionized form and may require deprotonation (step a). The free amino group adds to the carbonyl group (step b) to form an intermediate known as a carbinolamine. It loses water (step c) to form an ^-protonated Schiff base, which may lose a proton (step d). All the reaction steps reach equilibrium, often quite rapidly. Because the pKa of the protonated Schiff base is usually low whereas that of a protonated primary amino group is high, the equilibria will be pH-dependent. Consider formation of a Schiff base by reaction of an unprotonated amine with a carbonyl compound to give an unprotonated Schiff base, with formation constant Kf at high pH. If the pKa value of the amine is 10 and that of the Schiff base is 5, it is easy to show that the formation constant for reaction of the protonated amine to form protonated Schiff base at low pH, eg, below pH 3, will be only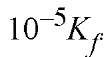 Consequently, the Schiff base will not be formed to a significant extent at low pH. If, however, the pK a of the amine is unusually low, or if the Schiff base proton is held by a hydrogen bond not present in the free amine, a Schiff base may be quite stable at neutral pH. This is the case for Schiff bases of pyridoxal phosphate.
Consequently, the Schiff base will not be formed to a significant extent at low pH. If, however, the pK a of the amine is unusually low, or if the Schiff base proton is held by a hydrogen bond not present in the free amine, a Schiff base may be quite stable at neutral pH. This is the case for Schiff bases of pyridoxal phosphate.
Schiff bases are chemically reactive, and various adducts can be formed. Especially popular is a reaction with a borohydride ion, which reduces the double bond of the Schiff base, converting the labile imine linkage to a stable secondary amine. This reaction has proved useful in linking pyridoxal phosphate to amino groups of enzymes or substrates, in attaching aldehydes to amino groups of receptors, and so forth (5, 6).
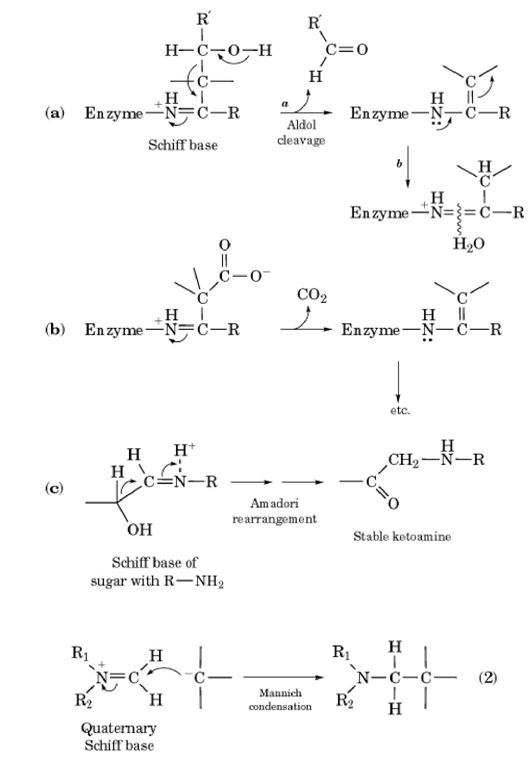
Schiff bases participate in many biological processes. In a common class of aldolases, protonated Schiff bases are formed with ketone substrates such as fructose 1,6-bisphosphate. The protonated imine serves as an electron-accepting group that facilitates carbon-carbon bond cleavage by the enzyme (Fig. 1a). (7, 8) A similar function is found for bacterial acetoacetate decarboxylase (Fig. 1b) (9). The Amadori rearrangement, prominent in carbohydrate chemistry, including nonenzymatic glycation and the Maillard browning reaction, also proceeds through a Schiff base mechanism (Fig. 1c). (10, 11) The Mannich condensation useful in organic synthesis (3, 12) is an addition reaction of a quaternary Schiff base, often derived from formaldehyde, with a carbanionic center (Eq. 2).
Figure 1. Participation of Schiff bases in three biologically important reactions: (a) type I aldolase; (b) beta-oxoacid decarboxylase, eg, acetoacetate decarboxylase; (c) Amadori rearrangement.