S1 nuclease is a 32-kDa nuclease that is specific for hydrolyzing of single-stranded RNA or DNA molecules into 5′ -mononucleotides. It is a zinc-requiring metalloprotein that is inactivated by chelating agents, such as EDTA and citrate, and by phosphate concentrations as low as 10 mM. Its enzyme activity is optimal at pH 4.0 to 4.3, halved at pH 4.9, and negligible above pH 6.0. The protein is thermostable (1) and resistant to several denaturing agents, such as urea, SDS, and formamide (2, 3). Digestion of single-stranded DNA is five times more efficient than that of single-stranded RNA, and 75,000 times more efficient than digestion of double-stranded DNA. The low level of cleavage of double-stranded DNA is minimized by increasing the ionic strength to about 0.2 M salt (2, 4-6), whereas it is increased by negative supercoiling of the double-helical DNA, UV irradiation, and depurination (3, 6-9).
Because S1 nuclease does not degrade double-stranded DNA or DNA-RNA hybrids, it is used widely to remove single-stranded regions from such duplexes. It is extremely useful for (1) measuring the extent of specific hybridization between single strands of DNA and/or RNA; (2) probing the existence of duplex DNA regions; (3) removing cohesive, sticky ends generated by restriction enzymes from single-stranded protruding DNA; (4) increasing the specificity of nucleic acid hybridization (10); (5) localizing intron, exon boundaries; (6) isolating of duplex regions in single-stranded viral genomes (11, 12); (7) probing strand breaks in duplex DNA molecules (2, 9, 13); (8) cleaving double-stranded regions that have lower duplex stability (14, 15); (9) localizing inverted repeat sequences (14, 15); (10) introducing deletion mutations at D loops in duplex DNA; and (11) mapping the genomic regions involved in interactions with DNA-binding proteins (16). Its most frequent use in mapping DNA sequences that encode RNA is described further here, because it exemplifies the potential and limits of the technique.
1. Mapping DNA sequences that encode RNA
A method for mapping RNA molecules onto the DNA templates from which they were transcribed was first described by Berk and Sharp (17, 18). The rationale is illustrated in Figure 1. An RNA transcript R is complementary to n nucleotides of the coding strand of a DNA, D. Hybridization of the denatured DNA and RNA leads to a hybrid duplex molecule that has single-stranded overhangs. These are removed by digestion with S1 nuclease. The size of the resulting DNA fragment, that is, the value of n, can be determined by subjecting it to electrophoresis on a polyacrylamide gel like those used in DNA sequencing. S1 nuclease does not remove the poly A track or the -cap of eukaryotic messenger RNA when they are base-paired, but the 5′ -cap structure, it has been thought, sterically hinders S1 digestion of single-stranded mRNA at the phosphodiester bond adjacent to the first nucleotide (19). When mapping is done with an end-labeled probe, one can determine the polarity and the map position of the RNA on the corresponding DNA sequences (19). This strategy is very useful for mapping the 5′ -end of mRNA transcripts, thereby identifying promoter sequences (Fig. 2), and mapping the exons in spliced eukaryotic mRNA (Fig. 3).
Figure 1. Rationale for S1-nuclease mapping
Figure 2. Mapping of 5′ -proximal promoter sequences. Promoter sequences localized upstream of the 5′ terminus of eukaryotic spliced RNAs can be mapped after hybridization of RNA preparations with a labeled DNA template (corresponding to the coding strand) that contains the putative promoter sequences (as suggested by DNase hypersensitivity, sequence analysis, etc.). In this example, a DNA fragment XYZ encodes an RNA complementary to sequence YZ. The promoter sequences are localized in X. The 5′ -end of the RNA delineates the X-Y junction. The first step consists of preparing a single-stranded, 5′ -labeled DNA probe (XY) that contains the 5′ -proximal promoter sequences and does not cover the 3-end of the RNA molecule. This conserves the 5 ‘ -labeled end (the dark sphere) after S1 digestion. Then, this DNA strand (top) is hybridized to the mRNA (bottom). The DNA and RNA overhangs are digested by S1 nuclease, and hybridized regions are protected. The S1- digested hybrid is denatured before to analysis by denaturing gel electrophoresis. Only the labeled DNA fragments are visualized by autoradiography. Left lane: control undigested labeled probe (XY); right lane: S1-digested sample in which only the protected Y region of labeled probe is protected. The length of the protected DNA fragment (Y) is smaller than that of the initial probe (XY). The difference in size (X) identifies the starting point (5 -end) of the RNA molecule.
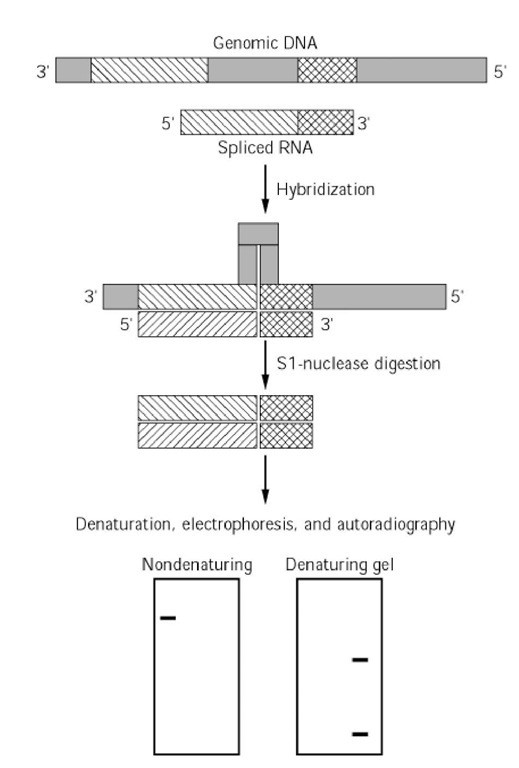
Figure 3. Mapping exon-intron junctions. In this example, a spliced mRNA composed of two exons is hybridized with the complementary, labeled DNA template (coding strand). Following hybridization of complementary sequences, a loop of single-stranded DNA (corresponding to the intron) is generated. Upon incubation of the partial DNA-RNA duplex with S1 nuclease, the free 3′ – and 5′ -proximal DNA overhangs and the loop are digested. The size of the resulting DNA-RNA hybrid, which contains only exon sequences, is determined by gel electrophoresis in a nondenaturing buffer. Establishing the individual sizes of the two exons requires gel electrophoresis under denaturing conditions to dissociate the hybrid strands. Only the labeled DNA fragments are observed by autoradiography.
Labeling the DNA fragmant
Determining the size of the S1-resistant DNA fragment relies on measuring a difference in size between the initial DNA probe and the protected segment. The DNA is usually detected specifically by autoradiography, which requires that it be radiolabeled and that the label be within the protected segment and survive the S1 nuclease digestion. Cloned DNA fragments are labeled uniformly or specifically at either the 5′ – or 3′ -termini. Labeling at the 5 ‘ -terminus is usually done with polynucleotide kinase in the presence of g- P-ATP, whereas 3′ -labeling is achieved with a P-nucleoside triphosphates and either the Klenow fragment of Escherichia coli DNA polymerase I, T4 polynucleotide kinase, or nucleotidyl terminal transferase.
2. Preparing single-strand templates
The previous labeling methods label both strands, so it is necessary to purify the labeled coding strand or to use conditions that favor hybridization between the RNA and DNA strands (see later). Generally, the best results are obtained with separated strands. After denaturation, the two strands of a DNA fragment may be separated by electrophoresis in polyacrylamide or agarose gels if they have different base compositions. It is not possible to predict how each strand will migrate, however, so the S1 mapping must be done with each strand. Hybridization of polyUG to the denatured strands may accentuate differences in their electrophoretic mobilities (20, 21). The double-stranded DNA fragment is digested asymmetrically from its two ends, so that the two single strands have different mobilities after denaturation (see Fig. 3). Alternatively, single-stranded DNA may be isolated by cloning in filamentous bacteriophage or plasmid vectors based upon such phage (see DNA Sequencing).
2.1. Use of Double-Stranded DNA Fragments
The rate of hybridization of RNA molecules to single strands of DNA is similar to that of DNA single-strand renaturation (22, 23). Therefore, the rate of hybridization is maximal in the presence of a high salt concentration (1 M NaCl) and a temperature corresponding to the melting temperature ™ of 25 to 30°C. In practice, hybridization is often done in the presence of formamide to decrease the tm and to hybridize at a lower temperature (24, 25). The melting temperature of duplex DNA molecules can be calculated (26) from the base composition, the ionic strength, and the formamide concentration by using the equation
At high concentrations of formamide (70 to 80%), the Tm of a DNA-DNA duplex is 5 to 10°C lower than the T m of the corresponding RNA-DNA hybrid (26). Under such conditions, a hybridization temperature between these two Tm values considerably favors association of the RNA with its coding DNA strand. Most RNA-DNA hybridizations are done at temperatures between 40 and 60°C with a buffer that provides constant pH and ionic strength, such as 0.04 M NaCl and 1 mM EDTA. To establish the optimal conditions for hybridization, the Tm of the DNA duplex used should be determined experimentally. Then, hybridization with the RNA is done at a temperature 2 to 4°C higher. The best results are usually obtained when excess DNA is used. Under such conditions, RNA-DNA hybridization is nearly complete in 3 hours with adenovirus 2 DNA (35 kbp) at a concentration of 10 |ig/mL (27). Extrapolating, it can be predicted that nearly complete hybridization of a DNA that has n kilobases will be obtained under similar conditions in 3 hours at a DNA concentration of (n/3.5) mg/mL.
In practice, the labeled, double-stranded DNA and the RNA molecule are mixed and reextracted together once. After resuspending the pellet in a "double-strand hybridization buffer" of 50 mM PIPES (pH 6.4), 1 mM EDTA, 0.4 M NaCl, 80% formamide, the mixture is incubated at 65°C to denature the nucleic acids. Then, it is transferred to the appropriate temperature for hybridization.
2.2. Use of Single-Stranded DNA Fragments
The probe and the target single-stranded DNA are resuspended in a "single-strand hybridization buffer" of 0.3 M PIPES (pH 6.4), 30 mM EDTA, 2.5 M NaCl, and incubated as before.


![tmp127-69_thumb[1] tmp127-69_thumb[1]](http://what-when-how.com/wp-content/uploads/2011/05/tmp12769_thumb1_thumb.jpg)
