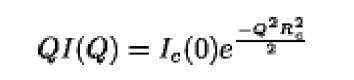The radius of gyration, R is a parameter that can be determined for biological molecules in solution using small-angle scattering techniques with X-rays, neutrons, or light. The R for a particle is defined as the root-mean-square distance of all elemental scattering volumes from their center of mass weighted by their scattering densities, and it is a simple but useful measure of the overall shape of the particle. Rg values for homogeneous geometrical shapes can be calculated analytically using the relationships in Table 1(1). These relationships can be very useful in interpreting experimentally determined Rg values. Table 2shows how Rg values vary for a single protein of a specific molecular weight and partial specific volume, assuming increasingly asymmetric shapes from a perfect sphere to a cylindrical rod. Also shown in Table 1are the Rg values for a six-subunit assembly assuming various arrangements of the subunits. It is readily seen that Rg gives a simple measure of the asymmetry in a particle. The mathematical formalisms used for determining Rg values from neutron and X-ray scattering data are somewhat different to those used for light scattering. We describe here the conventional equations used in the interpretation of neutron and X-ray scattering data. The formalisms used in light scattering are given in its own topic.
Table 1. Formula for Calculating Rg Values of Simple Geometric Shapes
|
Sphere radius R |
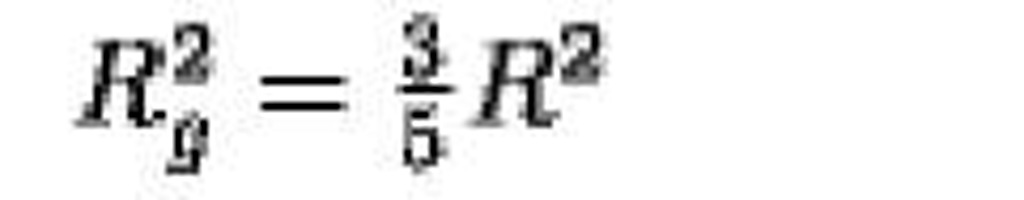 |
|
Hollow-sphere radii R1 and R2 |
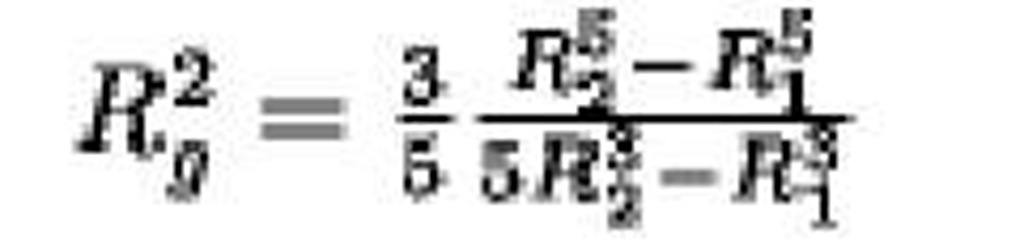 |
|
Ellipsoid, semi-axes a, b, c |
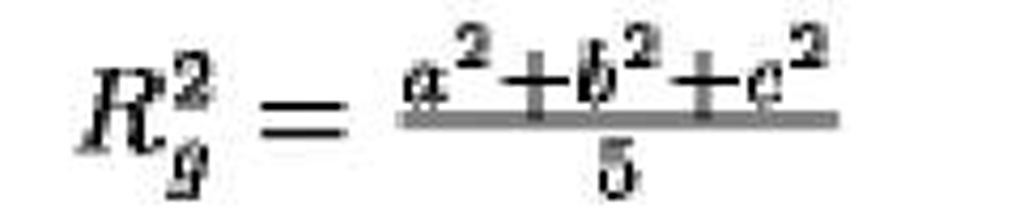 |
|
Prism, edge lengths A, B, C |
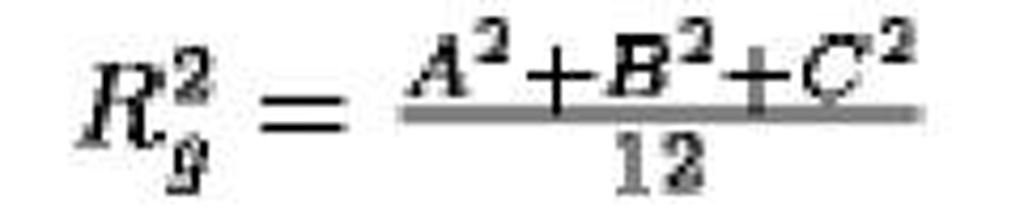 |
|
Elliptic cylinder, semi-axes a, b, height h |
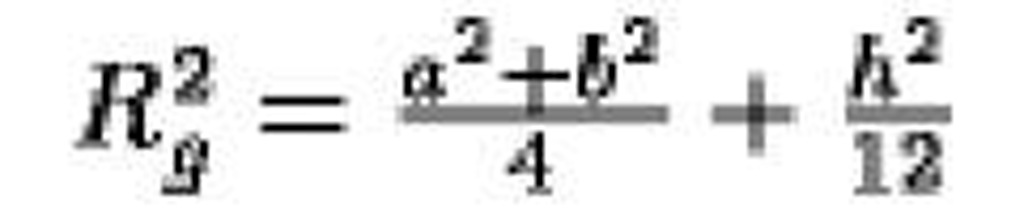 |
|
Hollow cylinder, height h, radii R^ R2 |
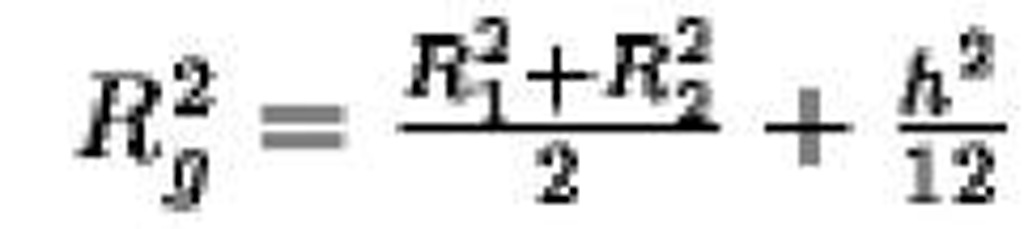 |
1. Determination of Rg from Small-Angle Scattering Data
The small-angle scattering of X-rays or neutrons yields the scattering intensity distribution, I (Q), which is related to the vector length or pair distribution function by a Fourier transformation:
Q is the amplitude of the scattering vector, which is equal to 4p(sine)/l (l is wavelength of the scattered radiation, and q is half the scattering angle). P( r) is the probable frequency distribution of all possible vector lengths, r, between scattering centers (or small volume elements) within a particle, weighted by the product of the scattering densities at the respective centers. Rg can be calculated as the second moment of the pair distribution function P(r):
Alternatively, one can determine Rg from scattering data using the Guinier approximation. In 1939 Guinier (2) showed that for sufficiently small Q-values:
A plot of log [I( Q)] versus Q2 thus yields Rg and the zero angle scatter, I(0), from the slope and intercept, respectively.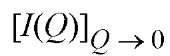 is directly proportional to the square of the molecular weight, M, of the scattering particle. For spherical particles, the Guinier approximation holds for Q<1.3/R and as the scattering particle becomes increasingly asymmetric, the approximation breaks down at even lower Q values.
is directly proportional to the square of the molecular weight, M, of the scattering particle. For spherical particles, the Guinier approximation holds for Q<1.3/R and as the scattering particle becomes increasingly asymmetric, the approximation breaks down at even lower Q values.
When one dimension of a particle is much greater than the other two (eg, a rod), Guinier (2) also showed that one can approximate the scattering for certain small- Q values as
where Rc is the radius of gyration of cross section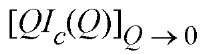 is directly proportional to the mass per unit length, Ml, of the scattering particle. For a rod of radius
is directly proportional to the mass per unit length, Ml, of the scattering particle. For a rod of radius![]() The range of Q for which equation (5) is valid depends on the shape of the cross section and the aspect ratio of the rod (3). For infinite cylindrical rods, equation (4) is valid for Q values <0.8/Rc. For finite rods there will be a "rollover" near the origin. Explicitly for rods of radius R and aspect ratio A = L/2R, the scattering intensity will decrease for values of Q<5/2AR that is, the higher the aspect ratio, the lower the value of Q where the rollover begins. For a rod-shaped object, Rg and Rc are related by
The range of Q for which equation (5) is valid depends on the shape of the cross section and the aspect ratio of the rod (3). For infinite cylindrical rods, equation (4) is valid for Q values <0.8/Rc. For finite rods there will be a "rollover" near the origin. Explicitly for rods of radius R and aspect ratio A = L/2R, the scattering intensity will decrease for values of Q<5/2AR that is, the higher the aspect ratio, the lower the value of Q where the rollover begins. For a rod-shaped object, Rg and Rc are related by
For particles with two dimensions much greater than the third (eg, a disk), the small Q scattering can be approximated as
where Rt is the radius of gyration of thickness. For a disk of thickness T, Rt = T/V12. Equations ( ) and (7) break down for particles with low axial ratios, and so they must be used with caution.
1.1. Changes in Rg Values Give Insights into Biological Function
In a series of scattering experiments on the dumbbell-shaped calcium-binding protein calmodulin, it was shown that upon binding a wide variety of amphipathic helices, calmodulin undergoes a dramatic conformational collapse involving its two globular lobes coming into close contact. The collapse is facilitated by a flexible helix linking calmodulin’s two globular domains, and it is characterized by an approximately 25% reduction in Rg (reviewed in Ref. 4). This conformational flexibility has proven key to understanding how calmodulin binds and activates a wide variety of target enzymes. Another example of the utility of determining R g values is the study by Mangel et al. (5) in which they characterized an extremely large ligand induced conformational change in native human Glu-plasminogen which was shown to have an Rg value of 39 A. Upon occupation of a weak lysine-binding site that regulates the activation of plasminogen, the protein’s shape irreversibly changes to give an Rg value of 56 A. This change in shape is achieved without any accompanying change in secondary structure, and the data have been interpreted in terms of a rearrangement of the five domains of the protein structure from a compact, closed structure that is relatively rigid, to an open flexible structure in which the individual domains no longer interact with each other and hence are more accessible. The increased flexibility in the open form is postulated to account for the observation that this form is more readily activated, because it would facilitate urokinase binding. The open form is also proposed to be key to plasminogen’s role in fibrinolysis.