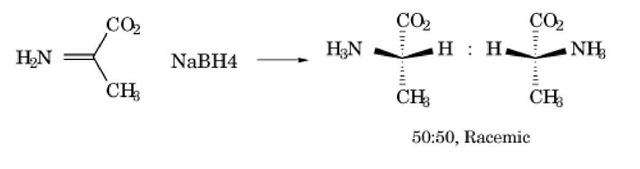
A mixture containing equal amounts of both enantiomers of a compound is a racemic mixture, which may also be called a racemate . The original compound identified as containing equal parts of two enantiomers was racemic acid as isolated from grapes (1). This compound is now known as (±) tartaric acid. When a chiral compound is formed by the reaction of two achiral reactants, the product must be a racemic mixture (2). An example of such a reaction is the reduction of the imine of pyruvate by sodium borohydride as shown in Figure 1. The product alanine is chiral, but both enantiomers must be generated in identical amounts.
Figure 1. Generating the chiral products l- and d-alanine from achiral reactants. The two products are produced in equal amounts, to give a racemic mixture of dl-alanine.
The process of separating a racemic mixture into its two enantiomeric components is called racemic resolution . Any process that catalyzes the interconversion of enantiomers, ie, a racemization , will necessarily result in a racemic mixture being formed. This must be so because the free energy of formation of the two enantiomers, in the absence of any other chiral compound, must be identical. During the chemical process of peptide synthesis, racemization of the activated amino acid derivatives has been a major problem. Enzymes, such as proline racemase, will racemize a solution of either the L-or D-enantiomer by catalyzing the interconversion of the two enantiomers, generating a racemic mixture of the substrate.