1. Introduction
Puromycin, first described in 1952, is a secondary metabolite of Streptomyces alboniger that blocks protein biosynthesis. Puromycin is a structural analogue of the 3′ end of aminoacyl-transfer RNA, but differs from tRNA insofar as the aminoacyl residue is linked to the ribose via an amide bond rather than an ester bond. Puromycin, like aminoacyl-tRNA, binds to the A site of the ribosome peptidyl-transferase center. When the A site is occupied by puromycin, peptidyl-transferase links the peptide residues of the peptidyl-tRNA in the ribosomal P site covalently to puromycin. Since the amide bond cannot be cleaved by the ribosome, no further peptidyl transfer takes place, and the peptidyl-puromycin complex falls off the ribosome.
Puromycin has facilitated the elucidation of ribosome structure and function, particularly the peptidyl-transferase reaction. The puromycin reaction provided the first demonstration that ribosomes can catalyze the formation of peptide bonds in the absence of cytoplasmic factors and without an added energy source, as well as the basis for distinguishing the A site from the P site (peptidyl-tRNA bound to the P site reacts with puromycin, whereas peptidyl-tRNA bound to the A site does not). The fragment reaction served to localize the peptidyl-transferase center on the 50S subunit of the bacterial ribosome.
2. Antibacterial Spectrum
In light of the structural resemblance of puromycin to aminoacyl-tRNA, it is not surprising that the spectrum of activity of this antibiotic is broad (1). The spectrum includes not only eubacteria and archaebacteria but also eukaryotes; consequently, puromycin is too toxic to be used clinically.
3. Structure-Activity Relationships
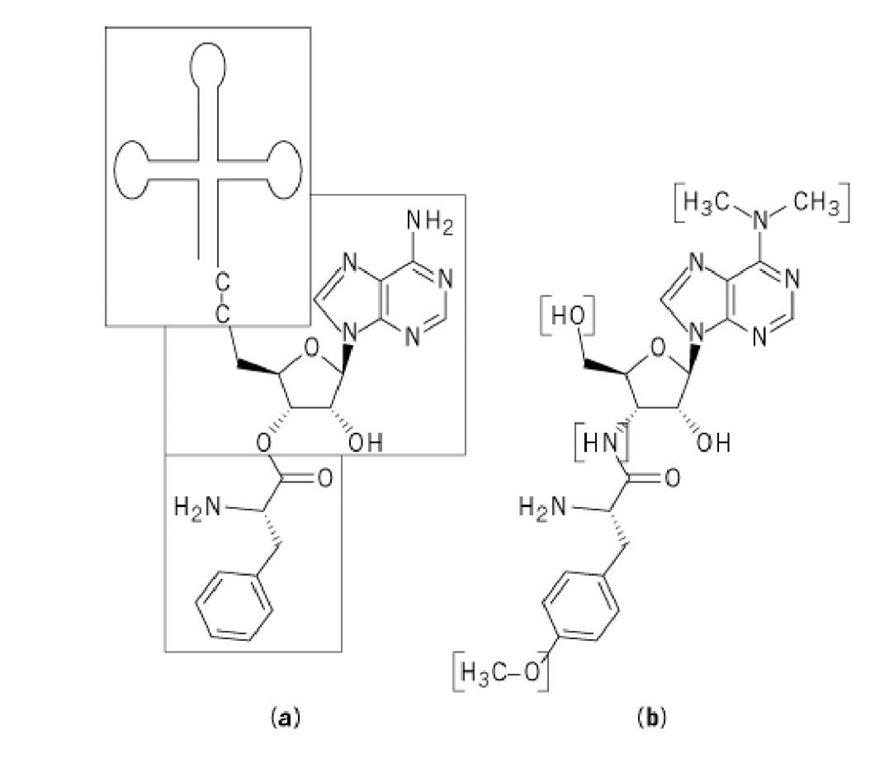
Puromycin essentially mimics aminoacyl-tRNA, in particular, phenylalanyl-tRNA (Phe-tRNA). The chemical structure of puromycin, 3′-(a-amino-p-methoxyhydrocinnamamido)-3′-deoxy-#,#-dimethyladenosine, is shown in Figure 1. In order for puromycin to retain its activity, both the nucleoside and amino acid moieties are required, and the amino acid moieties must be in the L configuration and have an unsubstituted amino group (i.e., the same requirement as that for amino acids incorporated into protein) (2). Derivatives with aromatic side chains are more active than aliphatic ones (3), which suggests that the aromatic aminoacyl residues may enhance the ability of puromycin to bind to the ribosomes.
Figure 1. Comparison of the chemical structure of puromycin and Phe-tRNA. (a) Puromycin mimics the amino acid part of Phe-tRNA (bottom box) and its 3′-terminal A (middle box). The tRNA body itself (top box) is absent in the puromycin molecule. (b) The differences between the two molecules are bracketed.
All tRNA molecules that are active in translation contain a conserved 3′-terminal sequence ending in an adenosine (A; see Transfer RNA). Similarly, puromycin retains the final A, although in a slightly modified form. Puromycin analogues having other nucleoside residues have little (I,C) or no (U,G) activity (4). The nucleoside and amino acid moieties of puromycin are linked by an amide bridge, rather than by an ester bridge as occurs in aminoacyl-tRNA. Since the amide bond is more stable than the ester bond, it cannot be cleaved by the ribosome.
4. Mechanism of Action
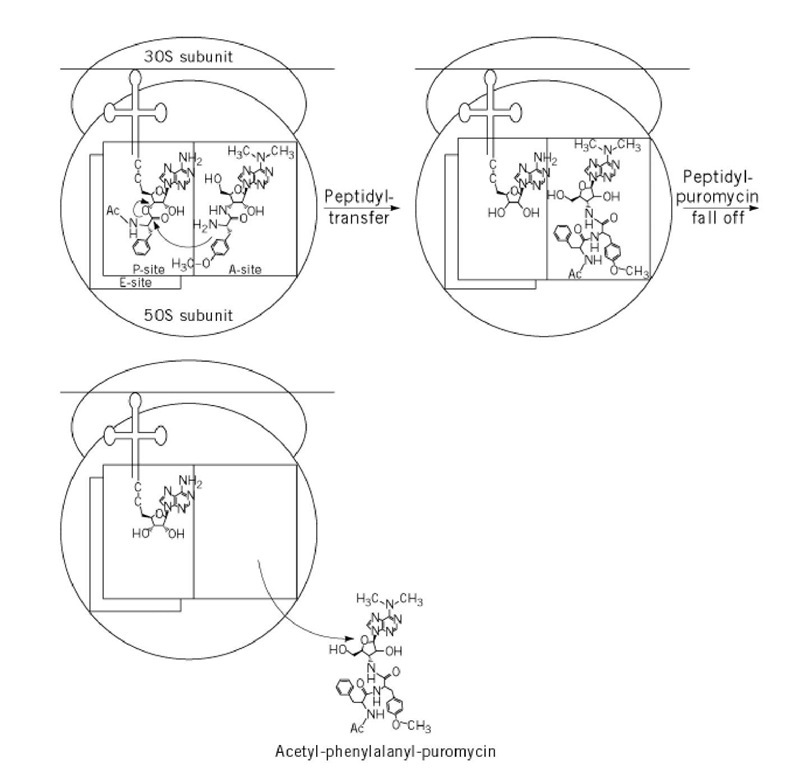
Puromycin blocks protein synthesis as follows (Fig. 2): first, puromycin binds to the A site of the peptidyl-transferase center of the ribosome in the pre-translocational state, where the P site is occupied by peptidyl-tRNA. Peptidyl-transferase then covalently links the antibiotic to the peptide residues of the peptidyl-tRNA in the P site. Because the ribosome cannot cleave the amide bond of puromycin and because peptidyl-puromycin, which lacks the whole tRNA body, has a lower affinity for the ribosome than peptidyl-tRNA, peptidyl-puromycin falls off the ribosome. High puromycin concentrations are needed to inhibit translation completely, because (i) the binding of puromycin to the ribosome is weak (^diss = 3 x 10-4 M) (5), (ii) one ribosome can transfer several puromycin molecules to peptidyl-puromycin, and (iii) once peptidyl-puromycin has fallen off the ribosome, it does not bind again and has no further antibacterial activity. The degradation of polysomes observed with puromycin in vivo and in vitro is caused by the release of peptidyl-puromycin from the ribosome, followed by ribosome subunit dissociation.
Figure 2. Puromycin reaction with P-site bound peptidyl-tRNA, eg, Ac-Phe-tRNA as the peptidyl-tRNA.
The puromycin reaction (6) is a classic in vitro experiment that has been used to explore ribosome function and to investigate the intrinsic peptidyl-transferase catalytic activity of the ribosome. This reaction, in which ribosomes are combined with puromycin and peptidyl-tRNA or its analogues (eg, Ac-Phe-tRNA), provided the first demonstration that ribosomes could catalyze the formation of peptide bonds in the absence of cytoplasmic factors and without an added energy source, such as GTP.
In the fragment reaction (7), 50S ribosomal subunits are combined with puromycin and a fragment of the tRNA molecule (CACCA-Leu-Ac) in the presence of 33% ethanol. Under these conditions, peptidyl-puromycin is still formed, demonstrating that the entire tRNA molecule is not necessary for peptidyl transfer. The fragment reaction also demonstrated for the first time that the entire ribosome is not required for peptidyl transfer and that the peptidyl-transferase center is located on the 50S subunit. Additionally, puromycin is used to investigate the inhibition mechanism of different translation inhibitors [eg, oxazolidinones (17) and azithromycin (18)].
The classic definitions of the tRNA binding sites on the ribosome are based on the ability of peptidyl-tRNA to react with puromycin: peptidyl-tRNA bound to the P site reacts with puromycin, whereas peptidyl-tRNA bound to the A site does not. The third tRNA binding site, the E site, is specific for deacylated tRNA, which does not undergo the puromycin reaction. Consequently, puromycin cannot be used to characterize this tRNA-binding site.
5. Ribosomal Binding Site
Our knowledge of the topography of the peptidyl-transferase center of the ribosome is largely derived from experiments with puromycin. In E. coli crosslinking experiments, a large number of proteins on the 50 ribosomal subunit, including L15, L18, L23, and L29, were labeled by puromycin and p-azidopuromycin (8-10). Most of these proteins are located in or next to the peptidyl-transferase center of the ribosome, and several can be affinity-labeled with other antibiotics that interact with this center (erythromycin, lincosamides, streptogramins, chloramphenicol). In contrast to the other peptidyl-transferase inhibitors, puromycin crosslinks proteins of both the large ribosomal subunit and the small subunit (eg, S7, S14, S18) (8-10). This indicates that the peptidyl-transferase center of the ribosome is located on the 50S subunit next to the 30S/50S subunit interface. P-azidopuromycin also cross-links nucleotides G2502 and G2504 of the central loop of 23 S rRNA (11) (see Peptidyl Transferase, Chloramphenicol); thus, this loop may be involved, directly or indirectly, in the peptidyl-transferase reaction (12).
6. Resistance
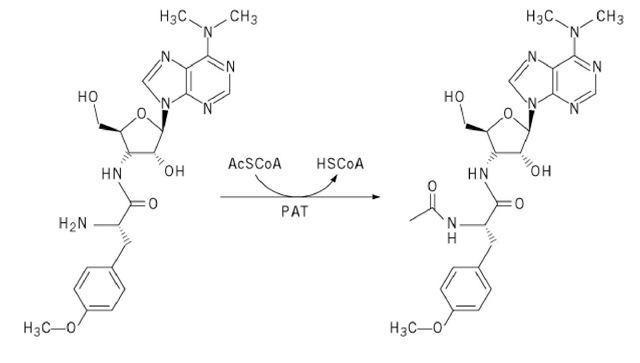
Puromycin can be inactivated by puromycin-N-acetyltransferase (PAT), which is found in the producing organism, Streptomyces alboniger (13). The resulting ^-acetyl puromycin (Fig. 3) cannot bind to ribosomes and fails to inhibit translation. Puromycin- #-acetyltransferase is commonly used as a dominant selectable marker for genetic studies of mammalian cell lines (14); the gene encoding this enzyme is used as a reporter gene in gene expression experiments (15).
In other organisms, efflux pumps have been identified that export puromycin from the bacterial cell.
Figure 3. Inactivation of puromycin by puromycin-^-acetyltransferase (PAT).
The genes encoding such pumps (eg, acrAB and envCD in E. coli and bmr and blt in Bacillus subtilis) confer a multiple-drug resistance phenotype (16).
So far, no resistance mechanism has been discovered that operates by ribosomal modification. This is not surprising, in light of the similarity between puromycin and tRNA: ribosomes that could not bind puromycin would probably be unable to bind tRNA and therefore be unable to synthesize protein.