The current literature uses the term progenote in two different ways: 1) it signifies an organizational level in evolution when prokaryotic organization preceded cells; or 2) it is used to denote the last common ancestor of all extant life. In some scenarios that describe early cellular evolution, it was assumed that the last common ancestor was at a preprokaryotic level of organization; however, subsequent analyses of the molecular evolution of different cellular components suggest that the last common ancestor was a prokaryote. Based on this realization, the term progenote should be more properly used to denote a hypothetical preprokaryotic stage in cellular evolution, distinct from the last common ancestor.
In 1977 Woese and Fox (1) defined progenote as a hypothetical stage in the evolution of cells where typical prokaryotic cellular organization preceded organisms:
Eucaryotes did arise from procaryotes, but only in the sense that the procaryotic is an organizational, not a phylogenetic distinction. In analogous fashion procaryotes arose from simpler entities. The latter are properly called progenotes, because they are still in the process of evolving the relationship between genotype and phenotype.
Woese and Fox intended to define an organizational level simpler than, and preceding, the prokaryotic level. At the progenotic level, genes and encoded proteins were smaller, and the accuracy of transcription and translation was lower than at the prokaryotic level. As a result, sequence evolution occurred more rapidly.
At the prokaryotic level, organisms contain a genome that encodes a multitude of biochemical and structural functions. Among the genome-encoded functions at the prokaryotic level are genome replication, translation of genomic information into functioning molecules, and formation of a semipermeable barrier between the organism and its environment. Prokaryotic organization is so complex that the likelihood is close to zero that a prokaryote spontaneously assembled from activated nucleotide and amino acid precursors in a primordial soup (see Prebiotic Evolution). A solution to this problem is to assume intermediate steps that successively evolve into more complex structures. An intermediate step usually assumed in the origin of life is self-replicating RNA-like molecules (see RNA World). However, because of the limited accuracy of these early replicators, the size and information contained in these self-replicating molecules is limited (2).
How a self-propagating network of biochemical reactions that maintains a boundary with the environment, that is, an autopoietic network (3), might have evolved from simple self-replicating molecules is a major puzzle in the evolution of life. Most scenarios that describe the evolution of cellular life include progenote-like organisms that are intermediate between the RNA world and the first prokaryotes [e.g., ((4); see Fig. 1b). However, the progenote concept that postulates an organism without strict coupling between genotype and phenotype, partially negates the major conceptual breakthrough associated with the RNA world, that Darwinian natural selection already acted on simple self-replicating RNA molecules. Several questions remain open regarding the evolution of progenotes: How does natural selection act on an organism without strict coupling between genotype and phenotype? Can progenotes evolve by natural selection without a phenotype encoded by their genes? Is it feasible that selection took place only (or mainly) at the level of individual molecules, not at the organismal level? Are alternative scenarios reasonable that assume tight coupling between genotype and phenotype at the level of self-replicating molecules that maintain this coupling throughout the transition stages from the RNA world to the prokaryotic level (2)
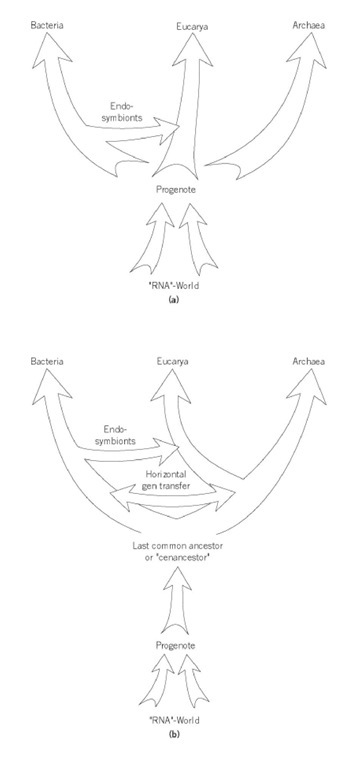
Figure 1. Comparison of two scenarios of the early evolution of cellular life: (a) the scenario envisioned when the term progenote was first defined (5, 6, 8); (b) the view that the last common ancestor was a prokaryote and that the progenote represents an earlier stage in the evolution of life (4, 10, 13).
At the same time that the term progenote was introduced to describe a pre-prokaryotic stage of cellular organization, an alternative meaning of the term progenote originated. Woese and Fox (1) argued that the last common ancestor of bacteria and the eukaryotic nucleocytoplasmic component was a progenote. Later, this argument was extended to also include a third line of descent, the archea or archebacteria ((5)-(7)). Woese and collaborators suggested that the three domains or Urkingdoms might have evolved independently from the progenote, that the optimization of the transcriptional and translational machinery occurred in parallel in the three lines of descent, and that the last common ancestor might be equally related to each of the three domains ((5)-(8)) (see Fig. 1a). Unfortunately, the initial definitions were ambiguous and potentially contradictory. Describing the evolution of bacteria and the eukaryotic nucleocytoplasmic component, Woese and Fox (1) summarize, The two lines of descent, nevertheless shared a common ancestor, that was far simpler than the procaryote. This primitive entity is called a progenote, to denote the possibility that it had not yet completed evolving the link between geno and phenotype.
Taken literally, this paragraph labels the ancestor of bacteria and eukaryotic nucleocytoplasm a progenote and justifies the choice of name by stating that the last common ancestor might have been at a preprokaryotic level of organization. As a result, the term progenote is often used in the sense of progenitor to denote the last common ancestor of archea, bacteria, and eukaryotes, not in the intended sense as a contrast to genote or eugenote, that is, organisms that have a "precise, accurate link between genotype and phenotype" (7).
Determining the properties of the last common ancestor is an important matter. Horizontal Gene Transferand the fusion of formerly independent lineages have turned the tree of life into a net of life (9). Characters found in all three cellular lineages might have been present in the last common ancestor, but these shared characters also might have evolved much later in one of the lineages and spread into the other two domains by horizontal transfer (10-12). The congruence of many molecular phylogenies supports the scenario depicted in Figure1b, and suggests that the last common ancestor had DNA and RNA polymerases, complex ribosomes made of both rRNA and proteins, and membranes already used for chemiosmotic coupling (13). However, if molecular phylogenies reflect horizontal gene transfer more than shared ancestry, recurring patterns might result from frequent gene transfers between some organisms and make the nature of the last common ancestor hard to discern. The use of ancient duplicated genes allows resolving the deep tripartite division of life into two successive bifurcations (14, 15). The current majority consensus considers that the archea is a sister group of the eukaryotes ((4, 13, 16); see Fig. 1b). According to this view (4, 13), the last common ancestor was a prokaryote that had a DNA genome, elaborate transcriptional and translational machinery, and strong coupling between geno- and phenotype. Although DNA replication and transcription appear to have been further optimized independently in the three domains (17), the last common ancestor appears to have been a prokaryote, not a progenote. Therefore, to avoid confusion, the last common ancestor of all extant life should be denoted the universal ancestor (8) or cenancestor (18), and the term progenote should be reserved to denote a hypothetical preprokaryotic stage in cellular evolution (1).