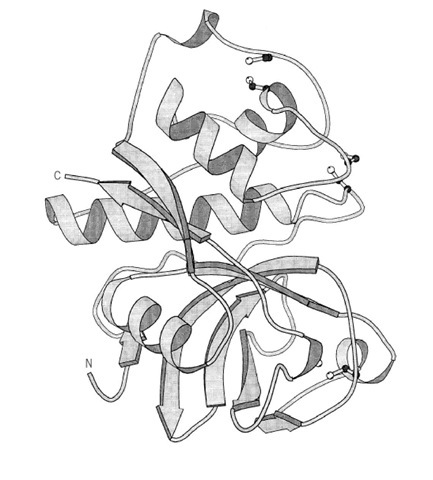Papain is a thiol proteinase that is obtained from the latex of the papaya tree (1) (Fig. 1). It has an essential cysteine residue at its active site that must be in the reduced form in order to be active. For this reason, it is often used in the presence of a reducing agent, such as b-mercaptoethanol or dithiothreitol. Papain has broad specificity and will hydrolyze peptide bonds adjacent to almost any amino acid residue, although it shows a trypsin-like preference for arginine and lysine residues. Its activity is optimal around pH 6. It is inactivated by reagents that react with thiol groups, such as iodoacetate or iodoacetamide, as well as silver and mercuric ions and p-chloromercuribenzoate.
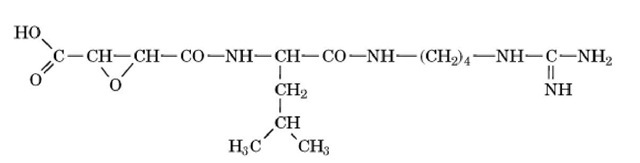
Other inhibitors include leupeptin, a peptide aldehyde that is a general thiol proteinase inhibitor, and E-64, an epoxide compound isolated from Aspergillus japonicus (Fig. 2). Leupeptin also inhibits some serine proteinases, whereas E-64 is more specific and has a strong affinity only for thiol proteinases. The epoxide group of the latter forms a thioether with the active site thiol group of the enzyme. It has little reactivity for thiol groups not in active sites.
Figure 1. The three-dimensional structure of the thiol proteinase papain. Only the polypeptide backbone is indicated schematically as a ribbon, with arrows for b-strands and coils for a-helices.
Figure 2. Chemical structure of E-64, an inhibitor of some thiol proteinases. Its chemical name is l- trans-epoxysuccinyl-leucylamide-(4-guanidino)-butane. The epoxy group forms a thioether linkage with the active-site thiol group of papain or cathepsin B.
Papain is used to degrade proteins in the presence of a reducing agent (2). Because it has broad specificity, it can generate many small peptides from a large protein. For this reason, it is usually employed to digest the intermediate-sized peptides that are generated by treating a protein with a proteinase of narrow specificity such as trypsin (see Peptide Mapping). Papain is used commercially in a popular brand of meat tenderizer.