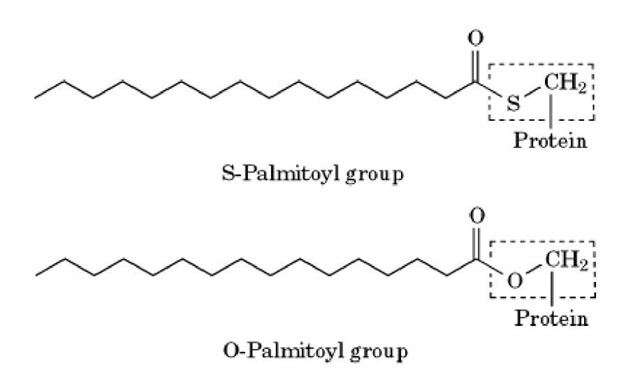
Palmitoylation is a post-translational modification process in which eukaryotic and viral proteins become ester- or thioester-linked to the 16-carbon saturated fatty acid, palmitic acid. Palmitoylation occurs on the thiol group of cysteine residues (S-palmitoylation) or on the hydroxyl group of serine and threonine residues (O-palmitoylation). S-palmitoylation (see Fig. 1) of cysteine residues close to the N- or C-terminus provides a membrane anchor for some cytosolic proteins, and is important for their correct localization within the cell. However, S-palmitoylation also occurs on polypeptide-anchored and integral membrane proteins, and its role in these proteins is uncertain.
Figure 1. Modification of a cysteine or serine residue by a palmitoyl group. An internal cysteine residue in the protein is thioester-linked to the 16-carbon saturated fatty acid, palmitic acid to give an S-palmitoyl group. Palmitic acid can also be ester-linked to serine (or threonine) residues to given an O-palmitoyl group. The cysteine and serine residue in each figure is indicated by a dotted line.
In contrast to the other lipid modifications of proteins (see Membrane Anchors), palmitoylation has proven relatively difficult to study. Even though palmitoylation was discovered more than 20 years ago and occurs on at least 50 different types of proteins, this process is still poorly understood at the biochemical level [for a comprehensive description of the early work in this area, see the review articles by Schmidt (1989) and Towler et al (1988)]. Apart from detailed studies on S-palmitoylation of cysteine residues in signal transduction components (eg, Src family tyrosine kinases, G-protein y subunits, Ras proteins, etc; see below), many of the initial reports of protein palmitoylation have not been substantiated in the last decade, and their functional significance is difficult to assess. There is essentially no recent information on O-palmitoylation of proteins. The lack of recent progress in these areas is due to several reasons.
1. For most of the "palmitoylated" proteins, the evidence for palmitoylation is based solely on the incorporation of H-labeled palmitate into cellular proteins followed by immunoprecipitation, SDS-PAGE, and autoradiography. It is usually possible to distinguish ester, or O-palmitoylation of serine and threonine from thioester or S-palmitoylation of cysteine in the H-labeled proteins by selective chemical deacylation procedures. However, more detailed analysis of H-labeled palmitoylated peptides is generally not practicable, because their unpredictable chromatographic behavior makes them very difficult to purify. This technical problem has thwarted attempts to identify the precise location of the incorporated H-label in most of the palmitoylated proteins. Indeed, for several "palmitoylated" proteins, it was subsequently shown that the incorporated palmitate is part of a completely different structure, the GPI anchor.
2. The 3H-labeled palmitate can be converted to other fatty acids before incorporation into some of the proteins. This suggests that the acyltransferase responsible for acylating the protein may be non selective and/or that different cells contain variable amounts of distinct acyltransferases with different specificities.
3. Protein S-palmitoyltransferase activities that can catalyze the transfer of a palmitoyl group from palmitoyl CoA to cysteine residues in protein or model peptide substrates have been identified in mammalian membrane fractions (1-5). Removal of the palmitoyl group by thioesterase activities has also been described. However, none of these activities has been thoroughly characterized at the molecular level, and it is difficult to tell if they are responsible for palmitoylation/depalmitoylation in vivo. Non enzymatic palmitoylation (autoacylation) of cysteine residues by palmitoyl CoA was also reported for several proteins, and this may account for some of the difficulties encountered in isolating and characterizing the protein S-palmitoyltransferase(s).
4. The criteria that determine which particular cysteine residue(s) in a protein will become palmitoylated are also uncertain. The presence of other lipid anchors (eg, prenyl or myristoyl groups; see below) on neighboring residues will facilitate cysteine palmitoylation presumably as a result of transient anchoring to membranes. These observations suggest that localization of "exposed" cysteine residence close to the bilayer surface, rather than a well-defined sequence motif, is the determinant for palmitoylation (4). S-palmitoylation of cysteine residues in polypeptide-anchored and integral membrane proteins could simply be the accidental result of proximity to the bilayer surface, where palmitoyl CoA and other molecular species of acylCoA are likely to be abundant.
As a consequence of the experimental problems identified above, there is relatively little information on the functional significance of palmitoylation for most proteins. However, site-directed mutagenesis studies, on a small number of proteins that are involved in signal transduction, demonstrated that a palmitoyl group can act as a membrane anchor and may play a role in regulating distribution of proteins between membranes and the cytoplasm as well as their association with lipid rafts (6) (for a general discussion of factors that can affect membrane affinity of lipid-anchored proteins, see Membrane Anchors). In some G protein y subunits and src-related tyrosine kinases, it has been shown that palmitoylation of cysteines near the N-terminus is dependent on prior myristoylation of the N-terminal glycine (7-9). Palmitoylation of y subunits can also be increased by receptor activation (10, 11). Similarly, palmitoylation of cysteine residues near the C-terminus of some Ras proteins is dependent on prior farnesylation (see Prenylation). Because farnesyl and myristoyl groups are both relatively weak anchors, the increase in membrane affinity resulting from palmitoylation has a major effect on the amount of these proteins bound to the membrane (12, 13). Furthermore, biophysical studies indicate that addition of palmitate not only increases binding affinity, but also increases the stability of membrane anchoring by reducing the rate of dissociation from the membrane (14, 15). Palmitoylation also occurs on cysteine residues in polypeptide-anchored proteins (eg, the transferrin receptor and CD4; see Table 1) and integral proteins with multiple transmembrane regions (eg, rhodopsin and some types of adrenergic receptor). As expected, preventing palmitoylation of these proteins by mutagenesis of the appropriate cysteine residues has no discernible effect on membrane anchoring. However, cysteine mutagenesis often has no, or relatively subtle, effects on the functional properties of the protein either, and the biological significance of palmitoylation remains uncertain in these cases.
Table 1. Examples of Palmitoylated Proteins
a Integral or polypeptide-anchored proteins. b O-palmitoylation; all other examples are S-palmitoylation.