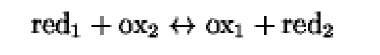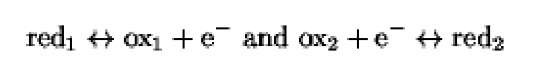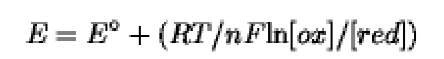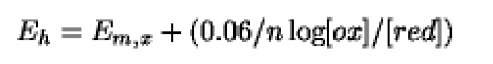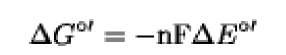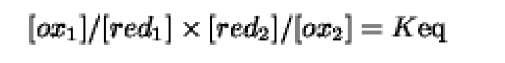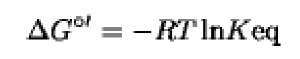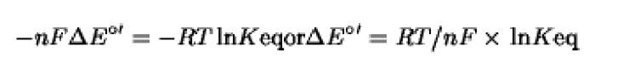Many of the fundamental physiologic reactions in a cell, both in the metabolic pathways of degradation and in the storage of energy via production of ATP, involve the transfer of electrons from one molecule, the donor (or reducing agent), to another, the acceptor (or oxidant). They are thus called redox reactions and are catalyzed by redox enzymes.
1. Redox Reactions and Redox Couples
The molecule donating an electron is the reductant (red) and becomes oxidized in the reaction, whereas the molecule accepting the electron is the oxidant (ox) and becomes reduced. In fact, each redox exchange:
is composed of two reactions:
The reductant and its corresponding oxidized form constitute a redox couple, in which both partners participate in the redox reaction. Each redox couple is characterized by a standard oxidation-reduction potential (pt) that quantitatively defines its tendency to loose an electron. In a redox reaction, the redox couple that has the higher affinity for the electron will be the oxidant, whereas the couple having a greater tendency to donate electrons will be the reductant. The same redox couple may therefore be either a reductant or an oxidant, depending on the redox potential of the second redox couple in the reaction. Examples of such a mechanism occur in the mitochondrial and the anaerobic bacterial electron transfer chains and in the photosynthesis pathway in chloroplasts, where the reductant of the (i)th step is the oxidant of the (i-1)th step.
2. Electrode Potential
The redox potential can be measured electrochemically as the electromotive force generated by connecting a half-cell of the redox sample with a reference half-cell. The reference half-cell consists of a platinum electrode immersed in a 1 M H+ solution and saturated with H2 gas at 1 atmosphere; the sample half-cell consists of an electrode immersed in a solution of the redox couple under standard conditions (1 M of the electron donor and acceptor, 25°C, and pH 7). The electrodes are connected to a voltmeter. A salt bridge (KCl solution) enables electrical continuity between the half-cells. The electrons flow in the direction determined by the redox potential of the sample relative to the reference. This is the voltage observed at the beginning of the experiment (standard concentration of 1 M), as the redox potential of the hydrogen electrode is arbitrarily taken as 0 V. The redox potentials of redox couples are thus expressed relative to the H2/2H+ couple.
For a redox reaction (ox + e- o- red), the Nernst equation relates the concentration of the redox species with the redox potential:
where E is the redox potential, E° is the redox potential for components in their standard state at pH 0, R is the gas constant ( 8.3144J K-1mol), F is the Faraday constant (9.65x104J V-1mol), n is the number of electrons transferred, and T is the absolute temperature. In biology, the equation is generally written as
where Eh is the redox potential referred to the standard hydrogen electrode, Em, x is the mid-point fl III’) a redox potential (when [ox] = [redj^standard state) at a defined pH (of x) and 2.303 RT/F = 0.06 for 29.4°C. The value of Em for a defined redox couple depends on the relative stability of the oxidized and reduced states: the more negative the value of Em, the more stable is the oxidized form and the stronger is the electron donor. Conversely, any factor stabilizing the reduced form makes the couple a better electron acceptor, having a more positive redox potential.
3. Redox Potential, Free Energy, and Equilibrium Constant
The change in free energy associated with a redox reaction (red1+ox2^ox1+red2) is related to the difference in redox potentials of the reactants by the formula:
in which n is the number of electrons transferred, F is the energy change as 1 M of electrons falls through a potential of 1 V ( 23.06kcalV-1mol-1), and DE°' is the difference in redox potentials of the reactants in volts. D G°' is the free energy change per mole and is expressed in kilocalories.
We know that at constant temperature and pressure a reversible reaction proceeds until an equilibrium is attained, defined by where R is the gas constant ( 1.987calmol 1K 1) and Tis the absolute temperature (K). We can also say that:
in which [ox^, [red^, [red2], and [ox2] are the reactant concentrations, and Keq is the equilibrium constant of the reaction at a certain constant temperature. Keq is related to the DG°’ free energy change by;
Knowing the redox potentials of a redox couple, the last relationships make it possible to calculate the equilibrium concentrations. Conversely, the redox potential of a solution can be calculated from the relative concentrations of the redox couples at equilibrium.
4. How to measure the redox potential
The oxidation of a reductant (red^ by an oxidant (ox2), at fixed temperature and pH, can be followed potentiometrically by measuring the variation of the electromotive force of a solution of the reductant as it is titrated with the oxidant. In practice, with a Pt electrode connected with a reference electrode (ie, a calomel electrode), the redox potential of the red1 solution is recorded after each addition of ox^. The redox potential increases as the value of log [ox^/[ red1] increases and is equal to Em when the initial reductant is half oxidized, [ox^ = [red^ = 50%. When [ox^ is close to 100%, the redox potential varies rapidly, and the titration comes to an end; the point of equivalence has then been reached, i.e. an equivalent of ox2 has been added to red^ In biological systems, the redox proteins generally carry colored groups that have substantial absorbance in the visible range of wavelengths. This allows precise determination by absorption spectroscopy of the relative concentrations of the oxidized and reduced species.
Many molecules can act as oxidizing and reducing agents in nonbiological redox reactions, whereas in the metabolic pathways of living organisms, a few molecules have been conserved during the evolution as redox agents for many different substrates.
The oxidation/reduction potentials of some biologically relevant compounds and free redox groups are presented in Table 1.
Table 1. Oxidation/Reduction Potentials of Some Biologically Relevant Compounds and Free Redox Groups
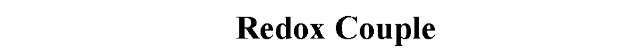 |
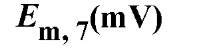 |
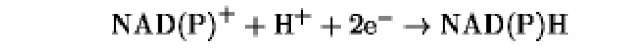 |
-320 |
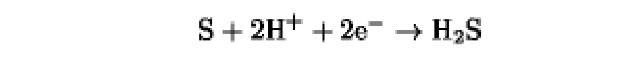 |
-230 |
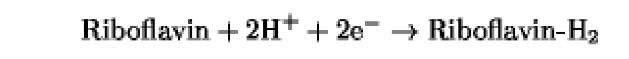 |
-200 |
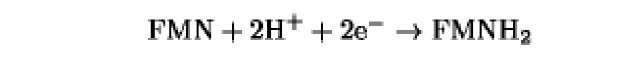 |
-219 |
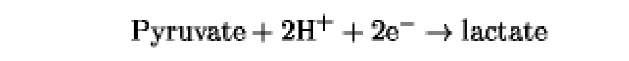 |
-190 |
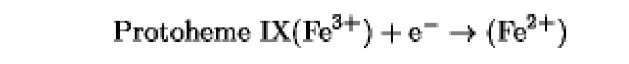 |
-115 |
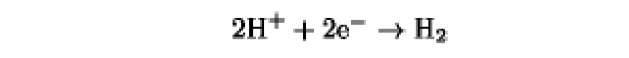 |
0.0a |
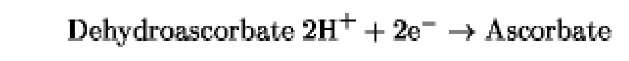 |
+60 |
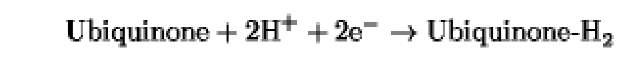 |
+100 |
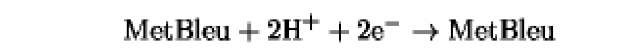 |
+110 |
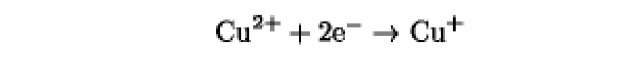 |
+150 |
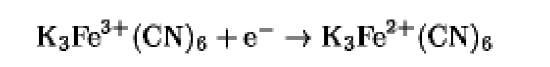 |
+360 |
|
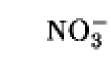 |
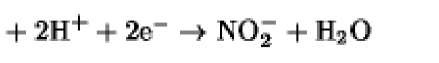 |
+480 |
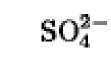 |
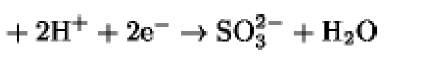 |
+480 |
 |
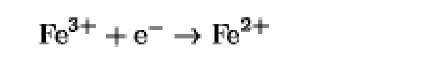 |
+770 |
 |
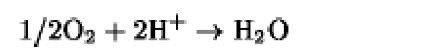 |
+820 |